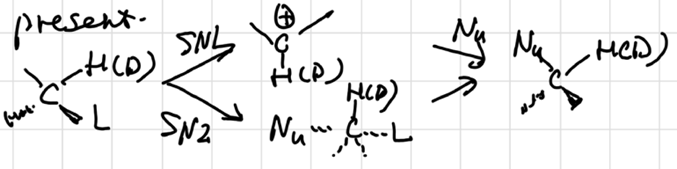Physical Chemistry II 物理化学 II 笔记
注意
此页面为物理化学II英文班的笔记,因此较为粗略。详细内容我之后会单开页面重新编写。
Chapter 1 : Chemical and Electrochemical Equilibrium Gibbs Energy Minimum for rxn \(A \rightleftarrows B\) , def. \(\xi\) as rxn extent (mol)
so \(-\dd n_A = \dd n_B = \dd \xi\) ,
\[ \dd G = \mu_A \dd n_A + \mu_B \dd n_B = (\mu_A-\mu_B)\dd\xi \]
for
\[ \Delta_rG = \dv{G}{\xi} = \mu_B - \mu_A = \mu_B^{\circ} - \mu_A^{\circ} + RT\ln{\frac{p_B}{p_A}} \]
def. rxn quotient \(Q = \frac{p_B}{p_A}\)
at Eq. \(0 = \Delta_rG = \Delta_rG^{\circ} + RT\ln{K}\)
General rxns \[ \nu_A A + \nu_B B \rightleftarrows \nu_C C + \nu_D D \]
we get:
\[ \Delta_rG = \Delta_rG^{\circ} + RTlnQ = \sum_j \nu_j \Delta_fG^{\circ}(j) + RT\ln(\prod_j a_j^{\nu_j}) \]
as \(a_j\) remains activity or fugacity
Since \(\delta _rG^{\circ}\) is defined as a single pressure, we have \((\frac{\partial K}{\partial p})_T = 0\)
for rxn \(A \rightleftarrows 2B\) , \(A\) changes from \(n\) to \((1-\beta)n\)
\[ x_A = \frac{(1-\beta)n}{(1-\beta)n + 2\beta n}, x_B = \frac{2\beta n}{(1-\beta)n + 2\beta n} \]
so we have:
\[ \begin{gathered} K = \frac{p_B^2}{p_A p^{\circ}} = \frac{x_B^2}{x_A} (\frac{p}{p^{\circ}}) = \frac{4\beta^2}{1-\beta^2}(\frac{p}{p^{\circ}}) \\ \beta = \frac{1}{(1+4 p/Kp^{\circ})^{1/2}} \end{gathered} \]
as \(p\) increase, \(\beta\) decrease.
Response of equilibria to changes as
\[ \ln{K} = -\frac{\Delta_rG^{\circ}}{RT} = -\frac{1}{R} (\frac{\partial \Delta_rG^{\circ}/T}{\partial T}) = \frac{\Delta_rH^{\circ}}{RT^2} \]
that is:
\[ \frac{\partial \ln{K}}{\partial {1/T}} = -\frac{\Delta_rH^{\circ}}{R} \]
that means: when \(\Delta_rH^{\circ} < 0\) , when \(T\) decrease, \(K\) increase. Vice Versa
Equilibrium electrochemistry for rxn \(\ce{H_2O <--> H_2 + O_2}\) we got half rxns:
Anode : \(\ce{H_2 - 2e^-<--> 2H^+}\)
Cathode : \(\ce{0.5O_2 + 2H^+ + 2e^- <--> H_2O}\)
for non-electrochemistry states:
\[ Q_1 = \frac{a(\ce{H^+})^2}{(p(\ce{H_2})/p^{\circ})}, Q_2 = \frac{a(\ce{H2O})}{(p(\ce{O_2})^{1/2}/p^{\circ})a(\ce{H^+})^2} \]
consider the Electromotive Force(EMF)
\[ \begin{aligned} -\nu EF = \Delta_rG &= \Delta_rG^{\circ} + RT\ln{Q} = \dv{W_e}{\xi}\\ E &= -\frac{\Delta_rG_m^{\circ}}{\nu F} - \frac{RT}{\nu F} \ln{Q} \end{aligned} \]
this is called Nerst Equation
At eq: \(0 = E^{\circ} - \frac{RT}{\nu F}\ln{K}\) , so
\[ \ln K = \frac{\nu FE^{\circ}}{RT} \]
Lattice gas def. \(N\) as indistinguishable particles , \(N_0\) as indistinguishable sites
as Boltzman Entropy\(S = k_B \ln{\Omega}\) , since \(\displaystyle \Omega = \frac{N_0!}{(N_0 - N)!(N)!}\)
let \(x = N/N_0\) , using Stiring Approximation
\[ S = -k_BN(x\ln{x} + (1-x)\ln{1-x}) \]
for general cases:
\[ S = -k_BN(\Sigma_n x_i\ln{x_i}) \]
Electrochemical Potential \[ G = H - TS + zeN\varphi \]
\[ \Delta G = \Delta H - T\Delta S + \Delta(zeN)\varphi \]
so as \(\displaystyle \mu = (\frac{\Delta G}{\Delta N})_{T,p,\varphi} = (\frac{\partial g}{\partial x})_{T, p, \varphi}\) , def \(g = G/N, h = H/N, S = S/N\)
For a lattice as:
\[ S' = (\frac{\partial S}{\partial x})_{T,p,\varphi} = k_B \frac{x}{1-x} \]
so:
\[ \mu = h' + k_BT\ln{\frac{x}{1-x}} + ze\varphi \]
in solution, when \(x \rightarrow 0\) ,
\[ \mu = \underline{h' + k_BT\ln{x}} + ze\varphi = k_BT\ln{\gamma x} + ze\varphi \]
that is called Electrochemical Potential
Standard Hydrogen Potential define Standard Hydrogen Potential as 0 V as the Standard Hydrogen Electrode :
\[ \ce{2H^+ + 2e^- -> H_2} \quad E^\circ = \pu{0V} \]
whereas happens Pt electrode and every species are at the standard state (形成铂黑结构增加催化位点,促进反应快速达到平衡。)
eg: \(\ce{2H_2 + O_2 -> 2H_2O}\) , \(E = \pu{1.23V}\)
so \(\ce{0.5O_2 + 2H^+ + 2e^- <--> H_2O}\) , \(E = \pu{1.23V}\)
eg2: \(\ce{AgCl + e <--> Ag + Cl^-}\)
\[ \begin{aligned} E &= E_{AgCl/Ag}^{\circ} - \frac{RT}{F} ln{a(\ce{Cl^-})} + \frac{RT}{F} ln{\frac{1}{a(\ce{H^+})}} \\ &= E_{AgCl/Ag}^{\circ} - \frac{RT}{F} \ln{a(\ce{Cl^-})a(\ce{H^+})}\\ &= E_{AgCl/Ag}^{\circ} - \frac{RT}{F} \ln{b(\ce{Cl^-})b(\ce{H^+})} -\frac{RT}{F} \ln{\gamma(\ce{Cl^-})\gamma(\ce{H^+})} \end{aligned} \]
where \(b\) stands molality (质量摩尔浓度), \(\gamma\) stands activity coefficient
as Debye-Huckle Formula\(\ln{\gamma} = cb^{1/2}\) :
\[ E = E_{AgCl/Ag}^{\circ} - \frac{2RT}{F} \ln{b} +\frac{2RT}{F} cb^{1/2} \]
Reversible Hydrogen Electrode (RHE) \[ E_{RHE}^{\circ} = E_{SHE} + \frac{RT}{F} \ln{a(\ce{H^+})}\\ V_{RHE}^{\circ} = V_{SHE} - \frac{RT}{F} \ln{a(\ce{H^+})} = V_{SHE} + 0.059\mathrm{pH} \]
Eg: Water Electrolysis
\[ \begin{aligned} V_{SHE} &= E^{\circ} - \frac{RT}{4F}\ln{\frac{1}{(p(\ce{O_2}/p^{\circ}))a(\ce{H^+})^4}} \\ &= E^{\circ} + \frac{RT}{F} \ln{a(\ce{H^+})} + \frac{RT}{4F} \ln{p(\ce{O_2})/p^{\circ}} \end{aligned} \]
where \(\displaystyle V_{RHE} = E^{\circ} + \frac{RT}{4F} \ln(p(\ce{O_2})/p^{\circ})\)
In real case, if we want to drive the rxn, need let \(E > E^{\circ} = 1.23 \mathrm{V}\)
Chapter 2 : Fuel Cells, Batteries and Electric Double Layer Determining TD functions \[ \begin{gathered} \Delta_rG^{\circ} = \Delta_rH^{\circ} - T\Delta_rS^{\circ} = -\nu FE^{\circ}\\ \frac{\dd E^{\circ}}{\dd T} = \frac{\Delta_rS^{\circ}}{\nu F} \to \Delta_rS^{\circ} = \nu F\dv{E^{\circ}}{T}\\ \Delta_rH^{\circ} = \Delta_rG^{\circ} + T\Delta_rS^{\circ} = -\nu F(E^{\circ} - T\dv{ E^{\circ}}{T}) \end{gathered} \]
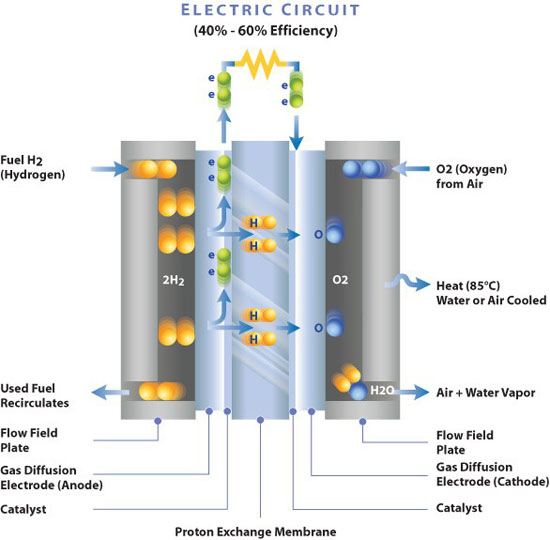
Energy Efficiency \[ \begin{gathered} \text{Fuel Cell}(EE) = \frac{\text{Electrical }E}{\text{Chemical }E}\\ \text{Electrolyzer}(EE) = \frac{\text{Chemical }E}{\text{Electrical }E} \end{gathered} \]
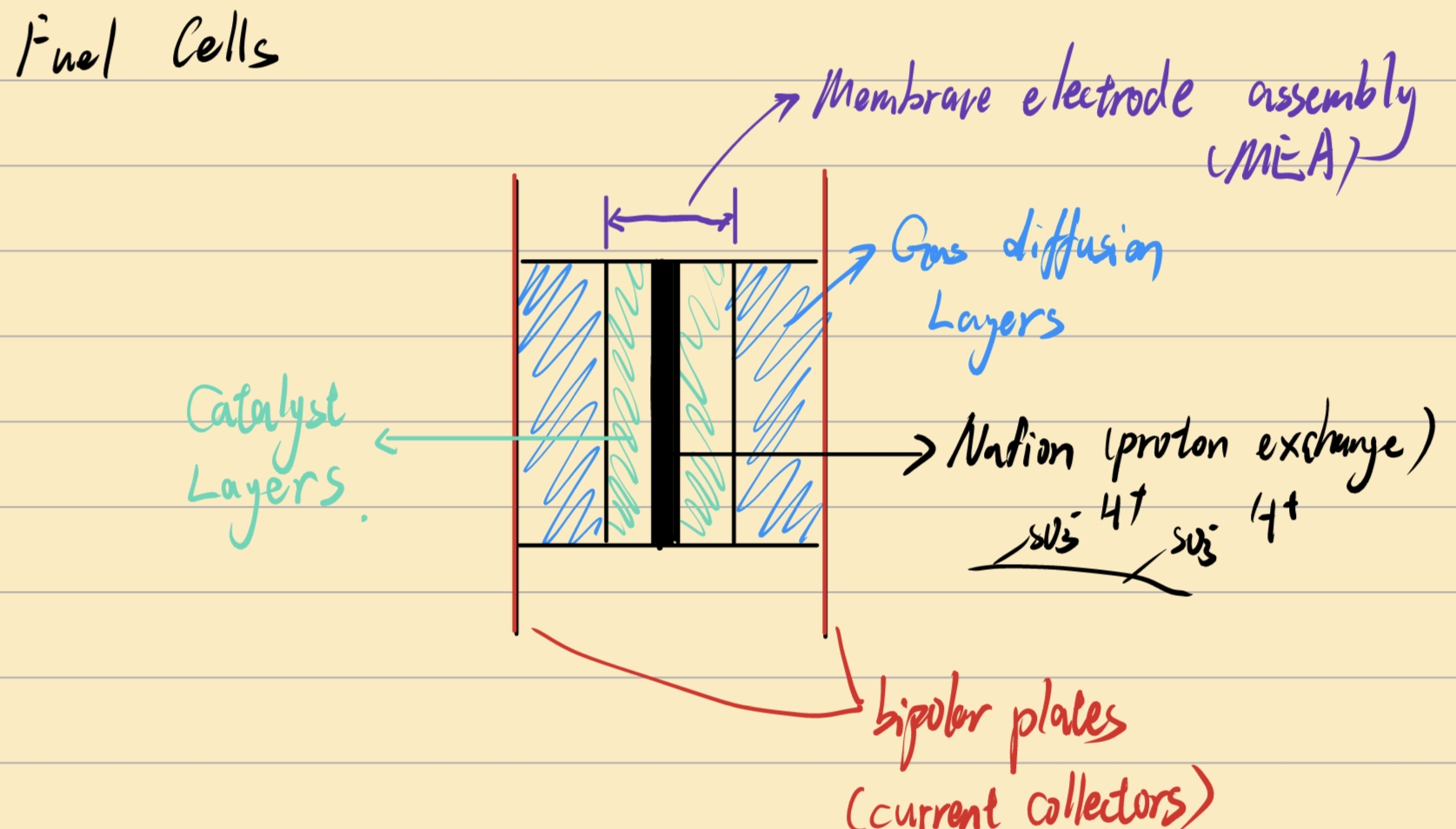
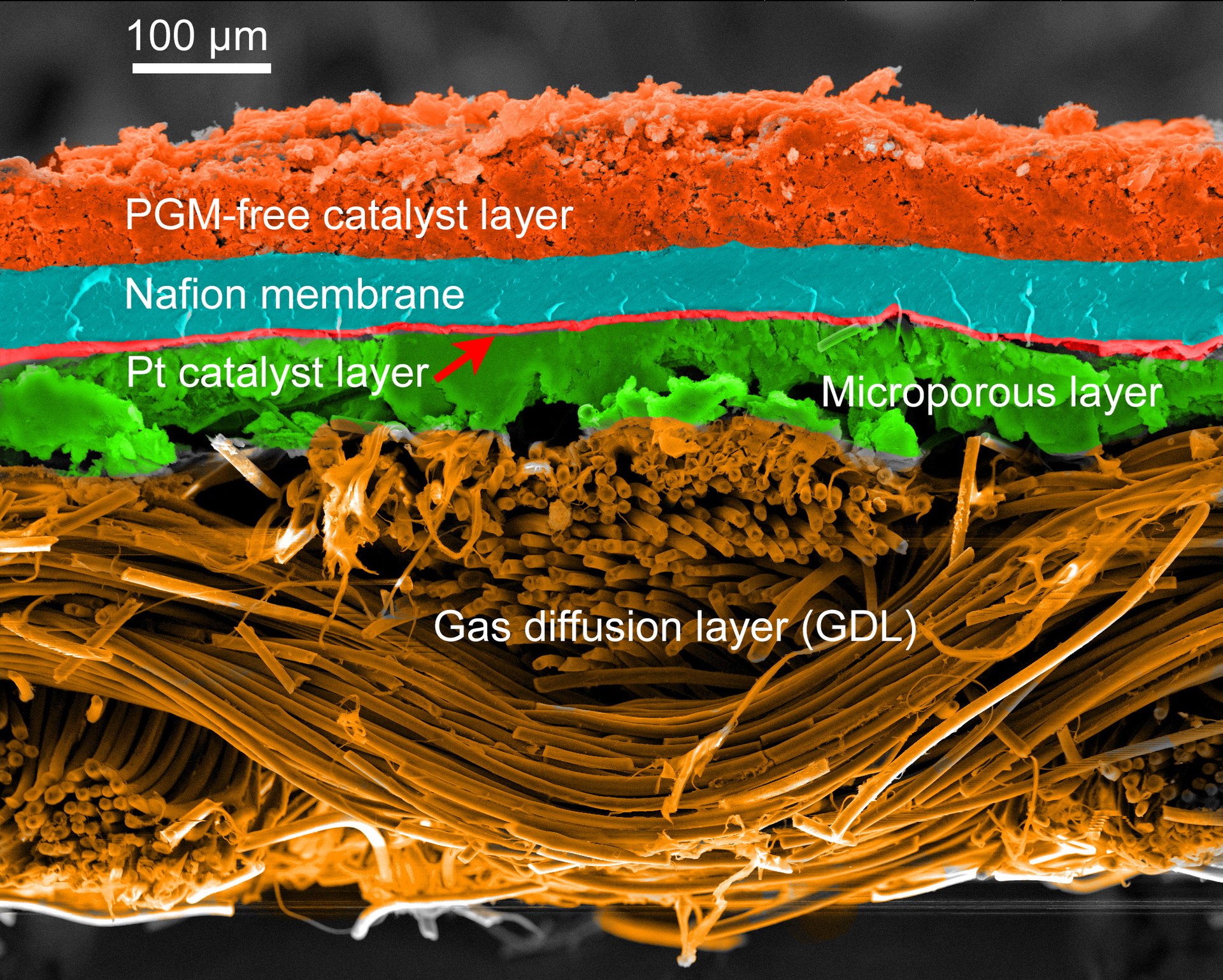
MEA: 膜电极组件
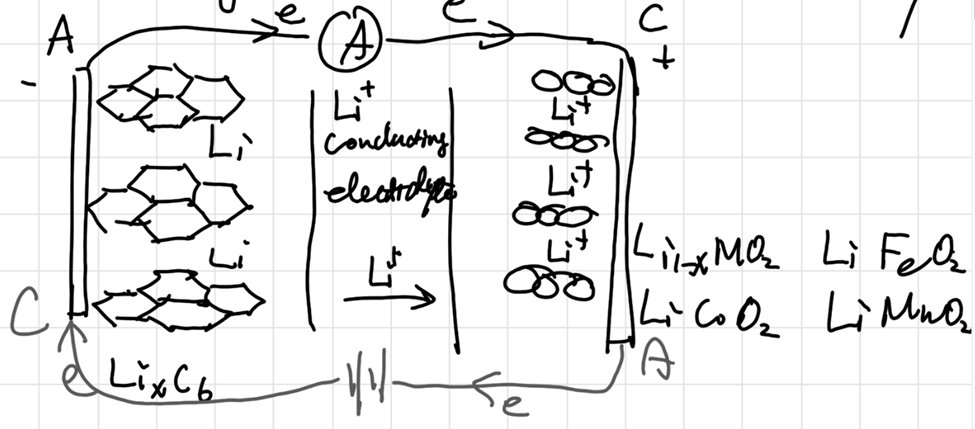
Rechargable batteries
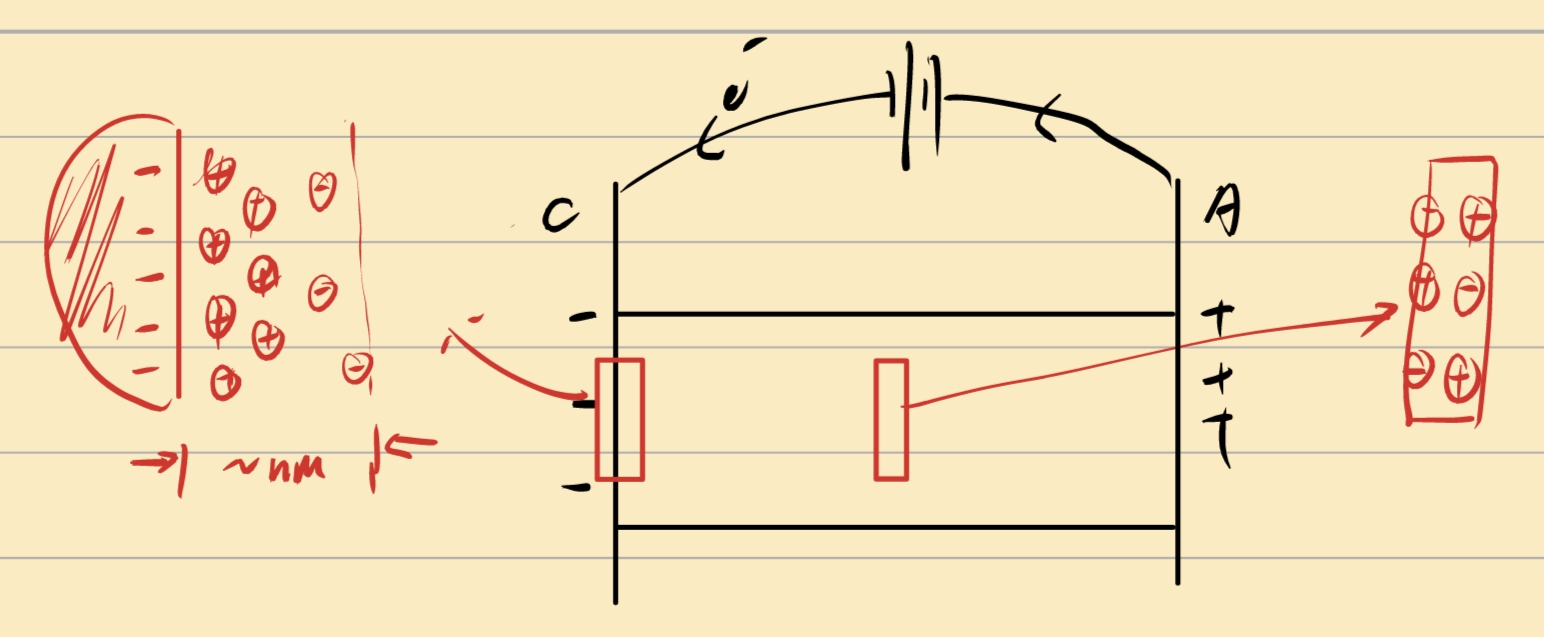
Charging Mode Positive Electrode : supply e- (Sluggish) Anode Negative Electrode : receive e- (Energetic) Cathode Electric Double Layer(EDL)
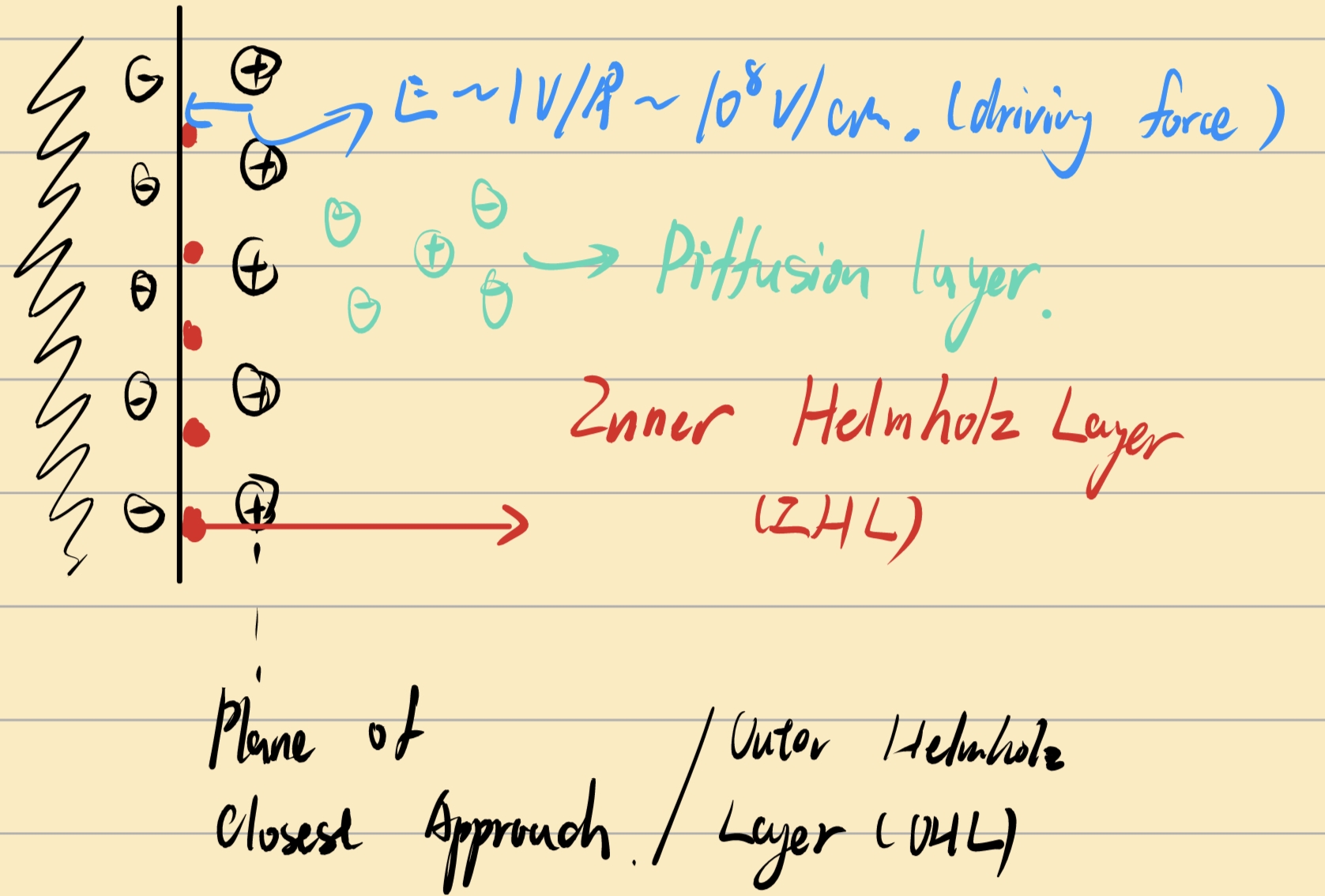
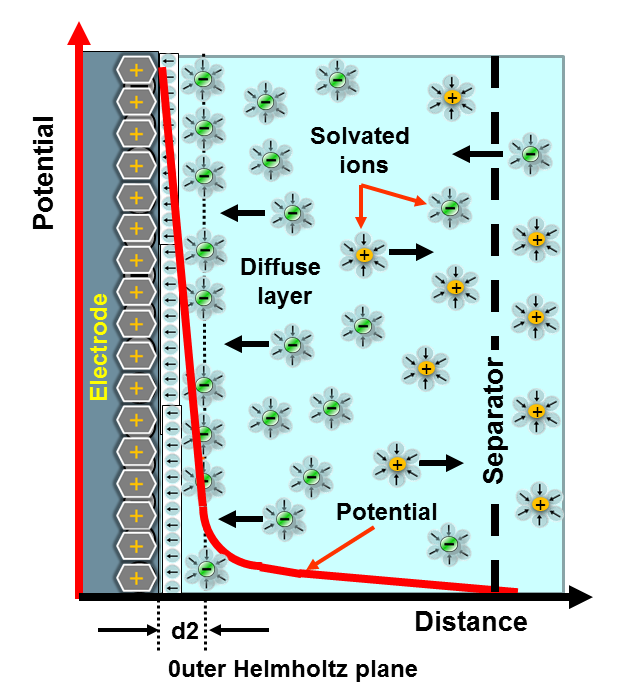
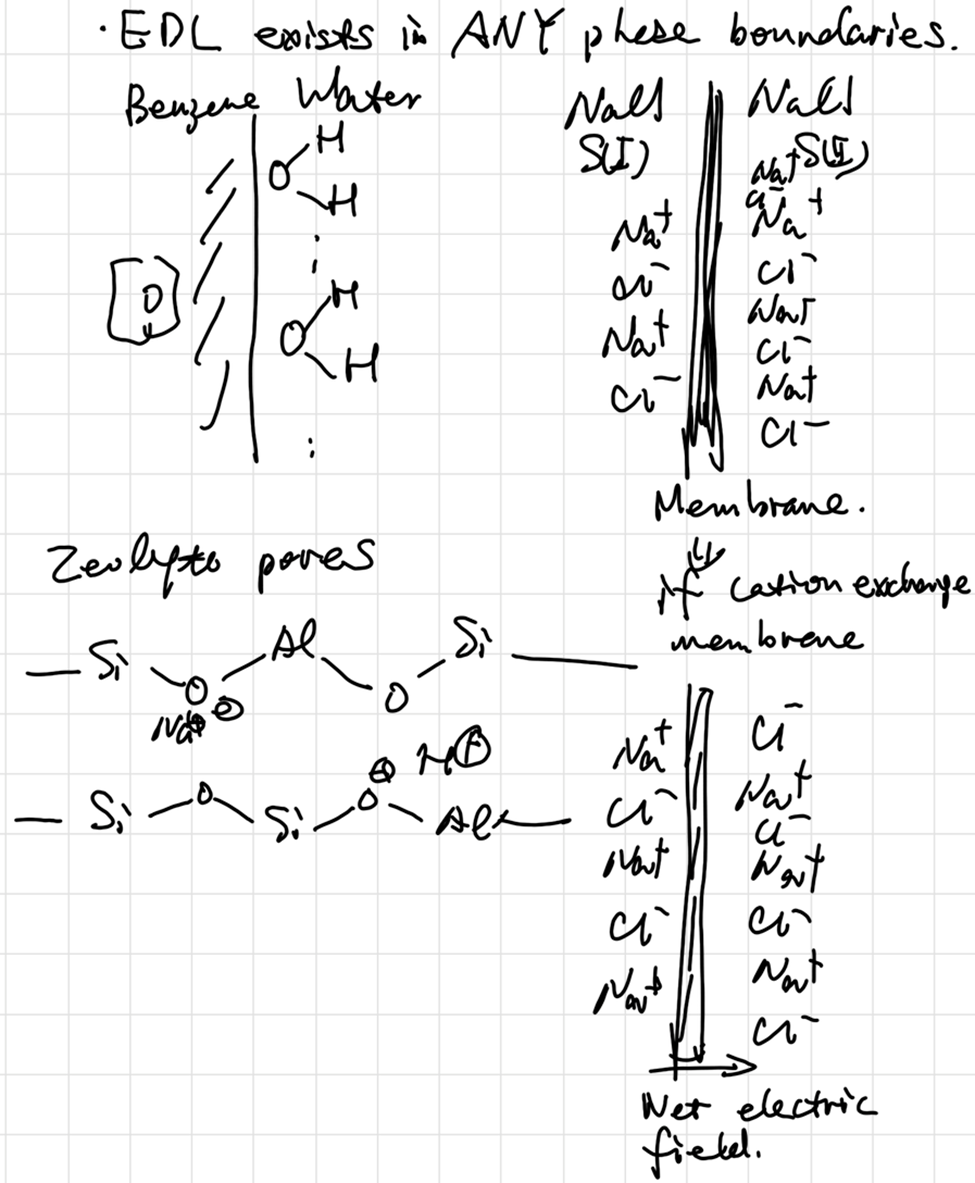
Gouy-Chapman-Stern(GCS) Model
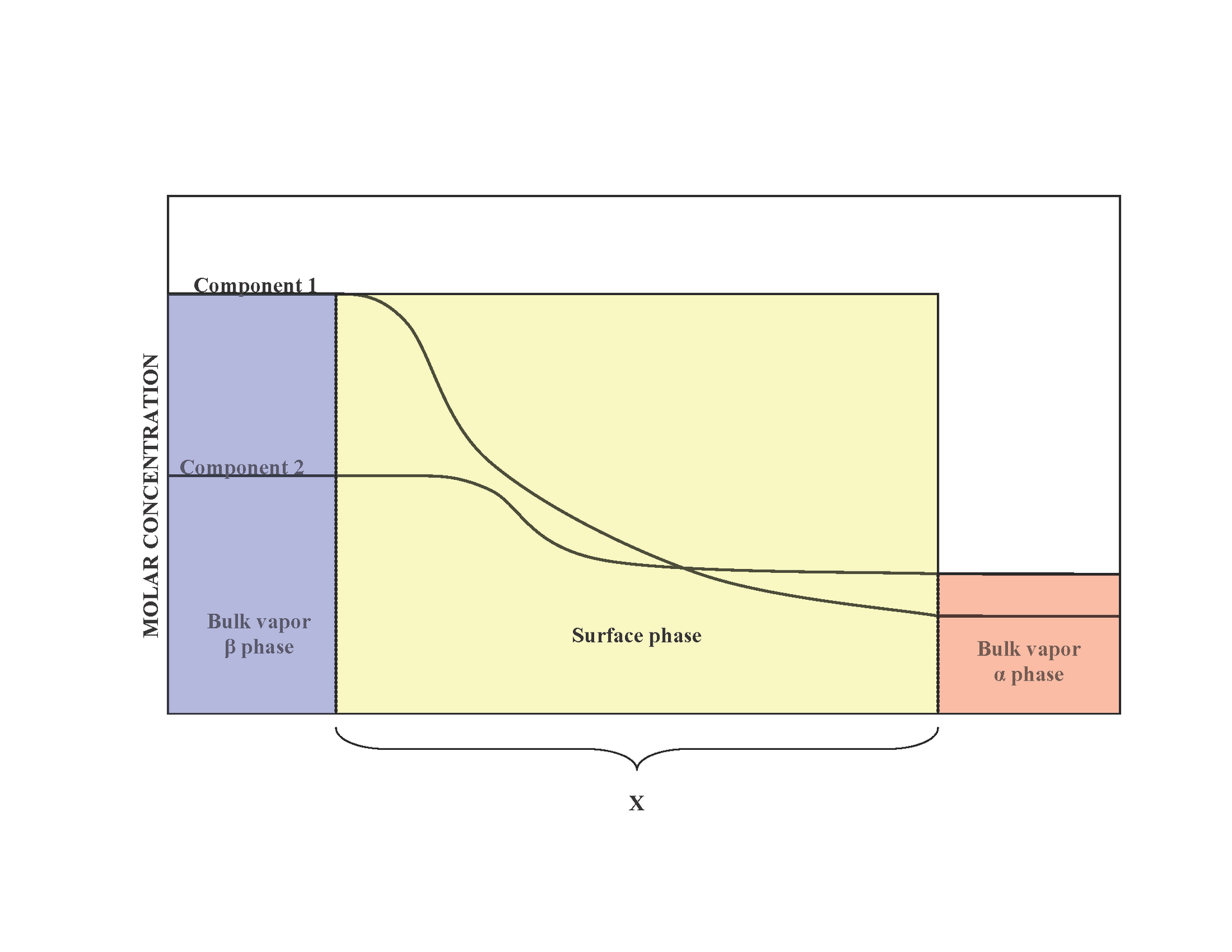
The Gibbs adsorption isotherm Pure Phase(A) | …… | Pure Phase(B)
Reference System: (省略了偏摩尔量)
\[ \dd G_R = (\frac{\partial G_R}{\partial T})\dd T + (\frac{\partial G_R}{\partial p}) \dd p +(\frac{\partial G_R}{\partial n})\dd n \]
Actual system:
\[ \dd G_S = (\frac{\partial G_S}{\partial T})\dd T + (\frac{\partial G_S}{\partial p}) \dd p +(\frac{\partial G_S}{\partial n})\dd n + (\frac{\partial G_S}{\partial A})\dd A \]
where \(A\) indicates interfacial area , we call \(\displaystyle(\frac{\partial G_S}{\partial A})_{p,T,n} = \gamma\) as Surface Tension
As p&T are const., 认为所有物质均为纯态,即\(\mu_i^R = \mu_i^S\) ,we have:
\[ \dd G^\sigma = \dd G_S - \dd G_R = \sum_i \mu_i(\dd n_i^S - \dd n_i^R) + \gamma \dd A= \sum_i \mu_i\dd n_i^\sigma + \gamma \dd A \]
\(n_i^\sigma\) is difference.
for total differential:
\[ \dd G^\sigma = \sum_i \mu_i\dd n_i^\sigma + \sum_i n_i\dd \mu_i^\sigma + \gamma \dd A + A \dd\gamma \]
that is Gibbs-Duham Relation :
\[ \begin{gathered} A\dd \gamma + \sum_i n_i^\sigma \dd \mu_i = 0 \\ -\dd \gamma = \sum_i \frac{n_i^\sigma}{A} \dd\mu_i = \sum_i \Gamma_i \dd\mu_i \end{gathered} \]
\(\Gamma\) is called Surface excess concertation (表面过剩浓度)
上文所说的实际上是,相对表面过剩浓度\(\Gamma_i^1\) ,即认为组分1的表面过剩为零的平面为划分面,其不依赖于选取的分界面的位置 :
\[ \Gamma^1_1 = 0, \quad \Gamma_i^1 = \Gamma_i - \Gamma_1 (\frac{C_i^\alpha - C_i^\beta}{C_1^\alpha - C_1^\beta}) = \Gamma_i - \Gamma_1 \frac{C_i^\sigma}{C_1^\sigma} \]
we have:
\[ \begin{gathered} \dd\mu_i = RT\dd \ln fC_i \\ \Gamma_i = -\frac{1}{RT}(\frac{\partial \gamma}{\partial \ln fC_i})_{T,p} \end{gathered} \]
Electrocapillary Equation \[ \ce{Wire---Cu'|Ag|AgCl(Ref)| K+, Cl-, M(electrolyte) |(WE)Hg|Ni|Cu---Wire} \]
for Working Electrode :
\[ \begin{aligned} -\dd \gamma &= (\Gamma_{Hg}\dd\mu_{Hg} + \Gamma_{e}\dd\mu_{e,Hg})\\ &+(\Gamma_{K+}\dd\mu_{K+} + \Gamma_{Cl-}\dd\mu_{Cl-})\\ &+(\Gamma_{M}\dd\mu_{M} + \Gamma_{H_2O}\dd\mu_{H_2O}) \end{aligned} \]
we have:
\[ \begin{cases} (\Gamma_{Hg}\dd\mu_{Hg} + \Gamma_{e}\dd\mu_{e,Hg}) = \Gamma_{e}\dd\mu_{e}^{Cu} \\ \dd \mu_{K+} + \dd \mu_{Cl-} = \dd \mu_{KCl} \end{cases} \]
for Ref Electrode :
\[ \ce{AgCl + e <--> Ag + Cl-} \]
\[ \mu_{\ce{AgCl}} + \mu_{e}^{Cu'} = \mu_{\ce{Ag}} + \mu_{\ce{Cl-}} \]
做全微分:
\[ \dd \mu_{e}^{Cu} = \dd \mu_{\ce{Cl-}} \]
代入原方程:
\[ \begin{aligned} -\dd \gamma &= \Gamma_{e}\dd \mu_{e}^{Cu} -(\Gamma_{K+} - \Gamma_{Cl-})\dd \mu_{e}^{Cu'} \\ & +\Gamma_{K+}\dd\mu_{KCl} +(\Gamma_{M}\dd\mu_{M} + \Gamma_{H_2O}\dd\mu_{H_2O}) \end{aligned} \]
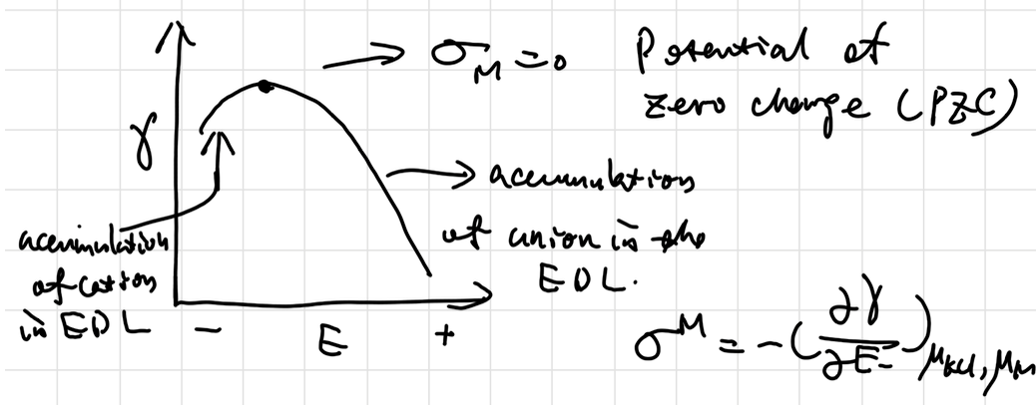
where \(\sigma_M = -F\Gamma_e\) , \(\sigma_S = -F(\Gamma_{K+} - \Gamma_{Cl-})\) stand for excess charge on WE and EDL
由于电中性:
\[ \sigma_M + \sigma_S = 0 \]
于是:
\[ \begin{aligned} -\dd \gamma &= -\sigma_M \frac{\dd \mu_{e}^{Cu}}{F} + \sigma_M \frac{\dd \mu_{e}^{Cu'}}{F} \\ & +\Gamma_{K+}\dd\mu_{KCl} +(\Gamma_{M}\dd\mu_{M} + \Gamma_{H_2O}\dd\mu_{H_2O}) \\ &= \sigma_M \dd E +\Gamma_{K+}\dd\mu_{KCl} + \Gamma_{M}\dd\mu_{M} + \Gamma_{H_2O}\dd\mu_{H_2O} \end{aligned} \]
由G-D方程:
\[ \dd \mu_{\ce{H2O}} = - \frac{x_{KCl}}{x_{H2O}} \dd \mu_{KCl} - \frac{x_{M}}{x_{H2O}} \dd \mu_{M} \]
代入:
\[ \begin{aligned} -\dd \gamma &= \sigma_M \dd E + (\Gamma_{K+} - \frac{x_{KCl}}{x_{H2O}})\dd\mu_{KCl} + (\Gamma_{M} - \frac{x_{M}}{x_{H2O}})\dd \mu_M\\ &= \sigma_M \dd E + \Gamma_{K}(H_2O)\dd\mu_{KCl} + \Gamma_M(H_2O)\dd \mu_M \\ &=\sigma_M \dd E + C \end{aligned} \]
that is Lippmann's equation :
\[ \boxed{\sigma_M =-\frac{\dd \gamma}{\dd E}} \]
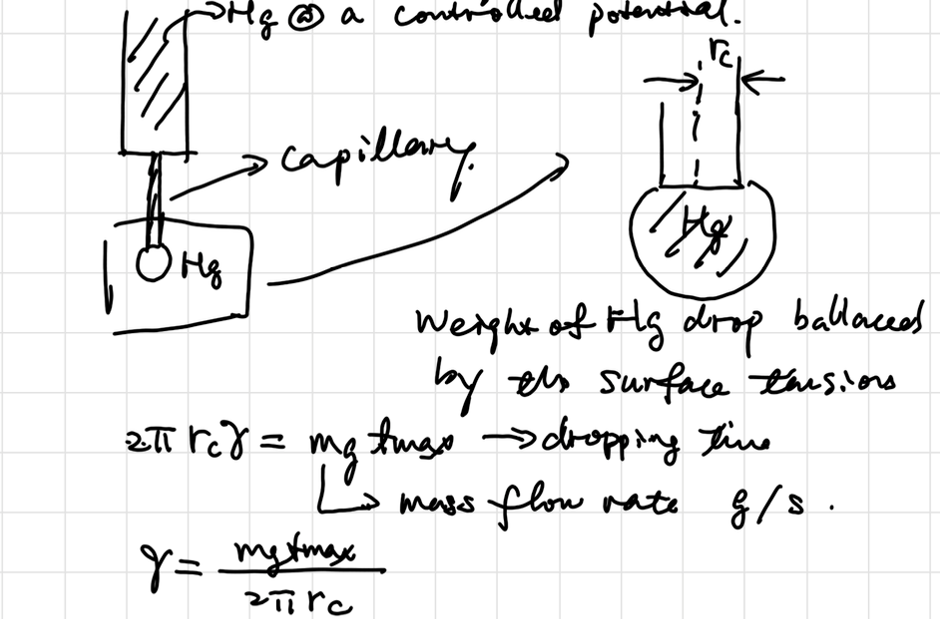
Droping Mercury Electrode
当且仅当导数为0时达到双电层净电荷平衡,前半段表面富集电子,排斥阴离子而吸引阳离子;后半段表面缺少电子,吸引阴离子。
Adsorption Isotherm \[ \begin{aligned} \mu_i^{Adsorption} &= \mu_i^{bulk}\\ \mu_i^{\circ A} + RT\ln a_i^{A} &= \mu_i^{\circ b} + RT\ln a_i^b\\ a_i^A &= a_i^be^{-\frac{\Delta G^\circ_i}{RT}} \end{aligned} \]
Assumptions:
All surface sites are identical No lateral interactions A full coverage can be achieved def \(a_i^A = \frac{\Gamma_i}{\Gamma_s-\Gamma_i}\) , \(\theta_i = \frac{\Gamma_i}{\Gamma_s}\)
that is:
\[ \begin{gathered} \frac{\theta_i}{1-\sum\theta_i} = a_i^be^{-\frac{\Delta G_i}{RT}}\\ \theta_i = \frac{a_i^be^{-\frac{\Delta G_i}{RT}}}{1+\sum_j^N a_j^be^{-\frac{\Delta G_i}{RT}}} = \frac{a_i^b K_i}{1+\sum_j^N a_j^b K_j} \end{gathered} \]
this is Langmuir Isotherm
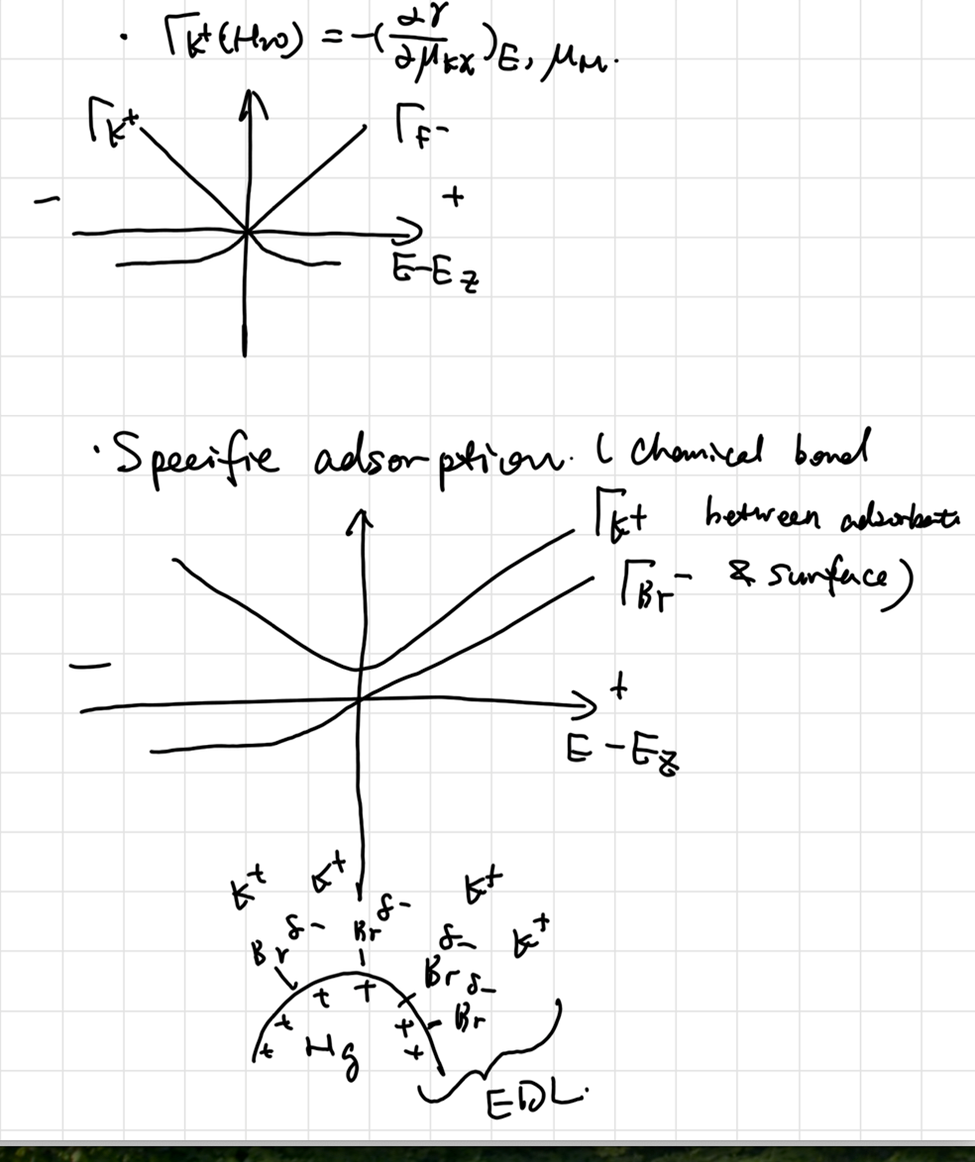
Including interaction among adsorbates : Frumkin isotherm
\[ a_i^be^{-\frac{\Delta G_i}{RT}} = \frac{\theta_i}{1-\sum\theta_i} \exp (-\frac{2g\Gamma_i}{RT}) \]
其中\(-2g\Gamma_i\) 代表相互作用项。
Chapter 3 : Chemical Kinetics Kinetic Theory of Gases KTG (Ideal Gases) \[ p = (\frac{\partial U}{\partial V})_S \quad \text{Pressure is a measurement of energy density} \]
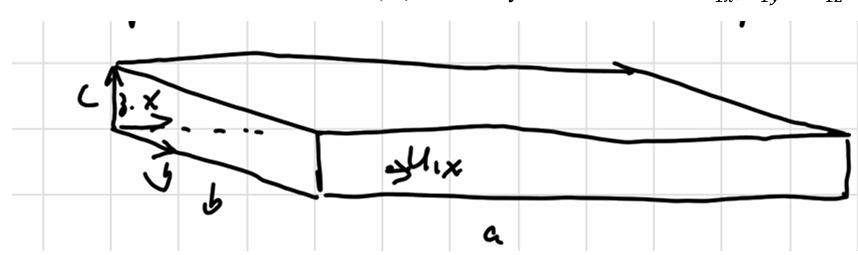
Assume one molecule in a \(abc\) box:
每次撞击动量改变为\(2mu\) ,间隔时间为:
\[ \Delta t = \frac{2a}{u_{1x}} \]
\[ \begin{gathered} F_1 = \frac{\Delta (mu_x)}{\Delta t} = \frac{mu_{1x}^2}{a} \\ P_1 = \frac{F_1}{bc} = \frac{mu_{1x}^2}{V}\\ P = \sum_i P_i = \frac mV \sum_i u_{ix}^2 \end{gathered} \]
With symmetry \(\langle u_x^2 \rangle = \langle u_y^2 \rangle = \langle u_z^2 \rangle\)
\[ \begin{gathered} u^2 = u_x^2 + u_y^2 + u_z^2 \rightarrow \langle u^2 \rangle = \langle u_x^2 \rangle + \langle u_y^2 \rangle + \langle u_z^2 \rangle = 3\langle u_x^2 \rangle \\ PV = \frac 13 Nm\langle u^2 \rangle \\ \frac 12m\langle u^2 \rangle = \frac 32 \frac{PV}{N} = \frac 32 k_BT \\ \frac 13 M\langle u^2 \rangle = RT \end{gathered} \]
that is :
\[ \boxed{\sqrt{\langle u_x^2 \rangle} = \sqrt{\frac{3RT}{M}}} \]
let \(h(u_x,u_y,u_z)\) be the fraction of molecules with velocity between \(u_x + du_x,u_y + du_y, u_z + du_z\) , as independent:
\[ h(u_x,u_y,u_z) = f(u_x) + f(u_y) + f(u_z) \]
之后利用Boltzman公式,然后归一化概率密度函数:
\[ \begin{gathered} f(u_x) = \sqrt{\frac{M}{2\pi RT}} e^{\frac{Mu_x^2}{2RT}}\\ h(u_x,u_y,u_z) = \left( \frac{M}{2\pi RT} \right)^\frac 32 e^{-M(u_x^2 + u_y^2 + u_z^2)/2RT} \end{gathered} \]
as polar coordinate,转化为球坐标(即在薄球壳内认为\(\times 4\pi u^2\) ):
\[ \begin{gathered} F(u)du = 4\pi \left( \frac{M}{2\pi RT} \right)^\frac 32 u^2 e^{-Mu^2/2RT} du\\ \boxed{ \langle u \rangle = 4\pi \left( \frac{M}{2\pi RT} \right)^\frac 32 \int u^3 e^{-Mu^2/2RT} du = \sqrt{\frac{8RT}{\pi M}} }\\ \langle u^2 \rangle = 4\pi \left( \frac{M}{2\pi RT} \right)^\frac 32 \int u^4 e^{-Mu^2/2RT} du = \frac{3RT}{M} \end{gathered} \]
求导可得最概然速率:
\[ u_p = \sqrt{\frac{2RT}{M}} \]
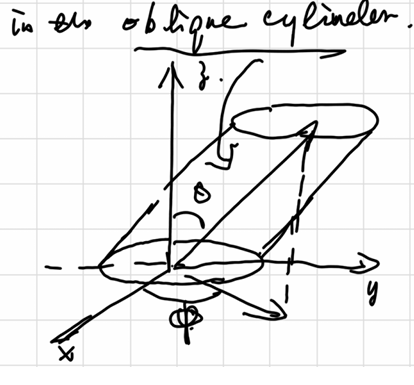
Collision of gas assume collision in \(dt\) in the oblique cylinder,考虑能撞上面积A的分子数:
柱形内碰撞分子数等于\(柱内分子数 \times 速度概率 \times 方向概率\)
\[ \begin{aligned} \dd N_{coll} &= \rho(Audt)\cos\theta \cdot F(u)du \cdot \frac{\sin\theta \dd\theta \dd\varphi}{4\pi} \\ \dd \xi_{coll} = \frac{\dd N_{coll}}{A \dd t} &= \rho u F(u)du \frac{\sin\theta\cos\theta \dd\theta \dd\varphi}{4\pi} \\ \xi_{coll} &= \frac{\rho}{4} \left< u \right> \end{aligned} \]
其中\(\xi_{coll}\) 代表碰撞频率,即单位面积单位时间的碰撞次数。
Mean Free Path
molecules in the cylinder will be collided
\[ \begin{gathered} dN_{coll} =\rho\pi d^2 \langle u_r \rangle dt \\ Z_A = \frac{dN_{coll}}{dt} = \rho\pi d^2\sqrt{\frac{8RT}{\pi \mu}} = \rho\pi d^2\sqrt{\frac{16RT}{\pi m}} \end{gathered} \]
(in dealing with two molecules relative motion)
\(Z_A\) is called collision frequency of one molecule
\[ l = \langle u \rangle dt = \langle u \rangle / Z_A = \frac{1}{\sqrt{2}\rho \pi d^2} = \frac{RT}{\sqrt{2}N_AP \pi d^2} \]
\(l\) is called mean free path
the total collision frequency \(Z\) is:(the average angle is 90° so \(u_r = \sqrt{2}u\) )
\[ \begin{gathered} Z_{AA} = \frac 12 \rho Z_A = \frac12 \pi d^2 \langle u_r \rangle \rho^2 = \frac{1}{\sqrt2} \pi d^2 \langle u \rangle \rho^2\\ Z_{AB} = \sigma_{AB} \langle u_r \rangle \rho_A \rho_B = \pi\left( \frac{d_A + d_B}{2} \right)^2 ·\sqrt{\frac{8RT}{\pi \mu}}·\rho_A \rho_B \end{gathered} \]
Collision theory : \(r \propto Z_{AB}\) and rxn only happen when \(u_r > u_0\)
\[ \begin{gathered} \dd Z_{AB} \propto u_rF(u_r) = u_r^3 \exp(-\frac{\mu u_r^2}{2k_BT}) \dd u_r = Au_rF(u_r) \\ Z_{AB} = A\int_0^\infty u_rF(u_r) \to A = \sigma_{AB}\rho_A\rho_B(\frac{\mu}{k_BT})^\frac32(\frac2\pi)^\frac12 \end{gathered} \]
let \(\epsilon_r = \frac12 \mu u_r^2\) :
\[ \begin{aligned} r = \int_{\epsilon_c}^\infty dz_{AB} &= \sigma_{AB}\rho_A\rho_B (\frac{1}{k_BT})^\frac32(\frac8{\pi\mu})^\frac12 \epsilon_r \exp(-\frac{\epsilon_r}{k_BT}) d\epsilon_r \\ &= \sigma_{AB}\rho_A\rho_B(\frac{8k_BT}{\pi\mu})^\frac12(1+\frac{\epsilon_C}{k_BT})\epsilon_r \exp(-\frac{\epsilon_r}{k_BT}) \\ &= A\exp(-\frac{\epsilon_r}{k_BT})\rho_A\rho_B \end{aligned} \]
即为Arrhenius Equation
Phenomenological Kinetics \[ \ce{\nu_AA + \nu_BB -> \nu_YY + \nu_ZZ} \]
\[ \begin{gathered} r = \frac{d\xi}{dt} = -\frac{1}{\nu_A}\frac{dn_A}{dt} = \frac{1}{\nu_Y}\frac{dn_Y}{dt} \\ r = k[\ce{A}]^{m_A}[\ce{B}]^{m_B} \\ \ln r = \ln k + m_A\ln{[\ce{A}]} + m_B\ln{[\ce{B}]} \end{gathered} \]
first/second order reactions ....
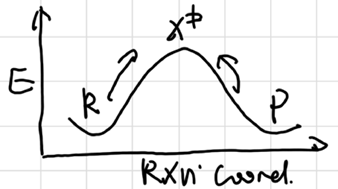
Transition State Theory :
Reactant are in eq. with the activated complex(AC) or transition state AC converts to product in a irreversible step \[ \begin{gathered} \ce{A + B <=> [AB]^\ddagger -> P} \\ K_c^\ddagger =\frac{[AB^\ddagger]c^{\circ}}{[A][B]} \end{gathered} \]
at eq \(\mu_A + \mu_B = \mu_{AB^\ddagger}\)
from statistical mechanics: \(Q = q^N / N!\)
\[ \begin{gathered} \mu = -RT(\frac{\partial \ln Q}{\partial N})_{VT} = -RT(\frac{\partial \ln {\frac{q^N}{N!}}}{\partial N})_{VT} = -RT\ln{q/N} \\ \mu^\ddagger = \mu_A + \mu_B \rightarrow \frac{q^\ddagger}{N^\ddagger} = \frac{q_A}{N_A} \frac{q_ B}{N_B}\\ \frac{N^\ddagger}{N_AN_B} = \frac{q^\ddagger}{q_Aq_B} \\ K_c^\ddagger = \frac{\frac{q^\ddagger}{V}·c_0}{\frac{q_A}{V}\frac{q_B}{V}} = \exp(-\frac{\Delta^\ddagger G ^\circ}{RT}) \end{gathered} \]
let \(\nu_c\) be the freq. at which AC cross over the barrier
\[ \begin{gathered} \frac{d[P]}{dt} = \nu_c[AB^\ddagger] = k[A][B] \\ k = \nu_c K_c^\ddagger /c^\circ \end{gathered} \]
Normal mode for the rxn 对于普通分子的三维配分函数:
\[ q_t = \frac{(2\pi mk_BT)^\frac32}{h^3} V \]
对于AC,只有一维的平动坐标:
\[ q_t^\ddagger = \frac{(2\pi m^\ddagger k_BT)^\frac12}{h} \delta \]
平衡常数
\[ \begin{gathered} K_c^\ddagger = \frac{(2\pi m^\ddagger k_BT)^\frac12}{h} \delta \frac{\frac{q^\ddagger_{other}}{V}·c_0}{\frac{q_A}{V}\frac{q_B}{V}} \\ k = \frac{(2\pi m^\ddagger k_BT)^\frac12}{h} \delta\nu_C \frac{\frac{q^\ddagger_{other}}{V}·c_0}{\frac{q_A}{V}\frac{q_B}{V}} = \frac{(2\pi m^\ddagger k_BT)^\frac12}{h} \left< u_{AC} \right> \frac{\frac{q^\ddagger_{other}}{V}·c_0}{\frac{q_A}{V}\frac{q_B}{V}} \end{gathered} \]
在一维坐标上积分速率得到
\[ \left< u_{AC} \right> = \sqrt{\frac{k_B T}{2\pi m^\ddagger}} \]
代入即可得到:
\[ k = \frac{k_BT}{hc_0} K^\ddagger = \frac{k_BT}{hc_0} e^{-\frac{\Delta G^\ddagger}{RT}} \]
Arrhenius Equation 求导得:
\[ \begin{aligned} \dv{\ln k}{T} &= \frac 1T + \dv{\ln K_C^\ddagger}{T} \\ &= \frac 1T + \dv{T}(\frac{\Delta G^\ddagger}{RT}) = \frac 1T + \frac{\Delta U^\ddagger}{RT^2} \\ &= \frac 1T + \frac{\Delta H^\ddagger + RT}{RT^2} = \frac{\Delta H^\ddagger + 2RT}{RT^2} \end{aligned} \]
由此即可得:
\[ E_a = \Delta H^\ddagger + 2RT \]
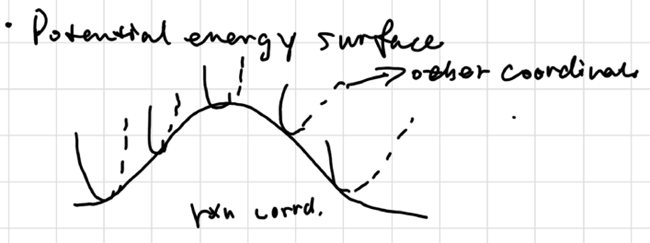
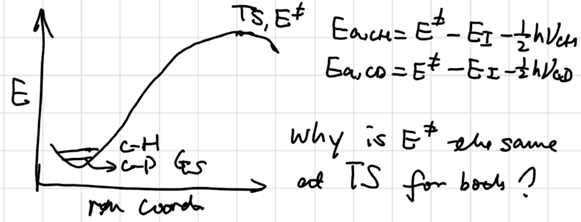
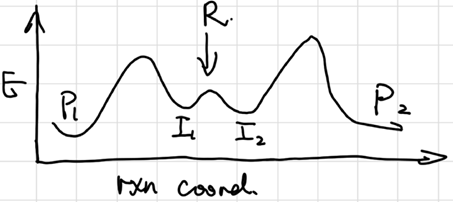
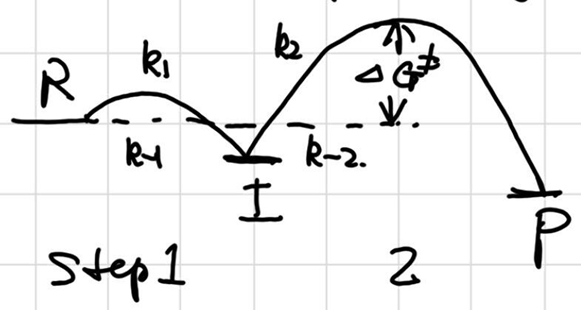
Chapter 4: Chemical Kinetics (II) Potential Energy Surface
the top point of the surface is called Saddle Point
at local minimum:
\[ \frac{\partial^2u}{\partial x^2} > 0, k>0, \nu = \sqrt{\frac{\mu}{k}} \text{ is real} \]
at local maximum:
\[ \frac{\partial^2u}{\partial x^2} < 0, k<0, \nu = \sqrt{\frac{\mu}{k}} \text{ is imaginary} \]
KIE Primary KIE Zero Point Energy : \(E_0 = \frac 12 h\nu\) for ground state
Harmonic Approx. : \(\displaystyle{\nu = \frac 1{2\pi}\sqrt{\frac k\mu}}, \tilde{v} = \frac 1{2\pi c}\sqrt{\frac k\mu}\)
\[ \frac {\nu_{CH}}{\nu_{CD}} = \sqrt{\frac{m_Dm_C(m_D+m_C)}{m_Hm_C(m_H+m_C)}} \approx \sqrt2 \]
\[ \begin{aligned} \ln(\frac{k'_{CH}}{k'_{CD}}) &= \ln{\frac {A_H}{A_D}} + \frac12 \frac{h(\nu_H-\nu_D)}{k_BT}\\ \frac{k'_{CH}}{k'_{CD}} &= \exp({\frac12 \frac{h(\nu_H-\nu_D)}{k_BT}}) \approx 1\sim10 \end{aligned} \]
Secondary KIE
KIE: \(S_N1\) ~ 1.2, \(S_N2\) ~ 1. why?
\(S_N1\) mechanism involves a temporary increase in the bond order of C-H(D)
Early vs. Late TS
Postulates based on TST Bronsted-Evans-Polanyi principle (BEP) for a series of similar reactions:
\[ \Delta G_1^\ddagger - \Delta G_2^\ddagger = \alpha(\Delta G_1-\Delta G_2) \]
or
\[ \ln(k_2/k_1) = \alpha\ln(K_2/K_1) \]
where \(0<\alpha<1\)
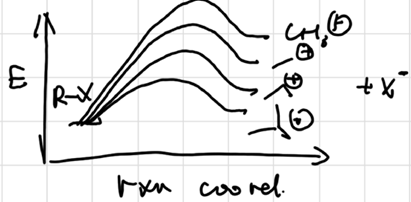
The Hammond Postulate
more reactive rxn will produce AC more resembles the reactant, result in less selectivity (Activity-Selectivity Principle )
The Curtin-Hammett Principle
The ratio of products is determined by the relative heights of the highest energy barriers, leading to different products
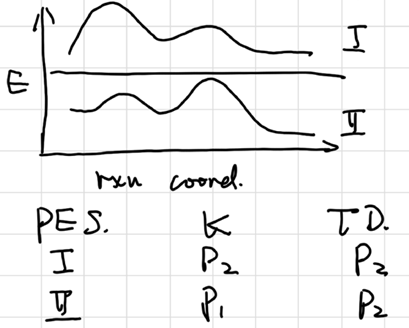
Microscopic Reversibility
The forward and reverse rxns go identical path
Kinetic vs Thermodynamic Control
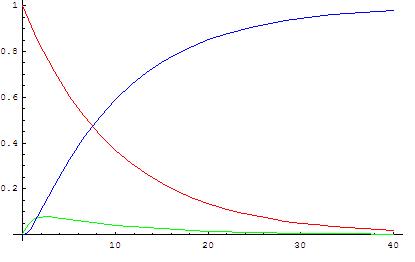
Quasi Steady Approx. (QSSA) Key assumptions:
consider rxn:
\[ \ce{A ->[k_1] B ->[k_2] C} \]
we have:
\[ \begin{gathered} \frac{dx}{dt} = -k_1x \quad \frac{dy}{dt} = k_1x-k_2y \quad \frac{dz}{dt} = k_2y \\ \Rightarrow y = \frac{k_1}{k_2 - k_1}(e^{-k_1t} - e^{-k_2t}) \\ \frac{dy}{dt} = 0 \Rightarrow t_{max} = \frac 1{k_2-k_1}\ln{\frac {k_2}{k_1}} \\ y_{max} = \frac {k_1}{k_2-k_1}[(\frac {k_2}{k_1})^{\frac {k_1}{k_2-k_1}} - (\frac {k_2}{k_1})^{\frac {k_2}{k_2-k_1}}] \end{gathered} \]
as \(k_2\gg k_1 \Rightarrow t_{max} \to 0, y_{max} \to 0\)
for rxns with more than one intermediates:
\[ \ce{A->[k_1]I_1 ->[k_2]I_2->...->I_n->[k_{n+1}]B} \]
all \(\ce{I_n}\) can consider into one steady state.
Pseudo-Equilibrium/ Rate limiting step If one step has a significantly lower rate constant, then it is considered as RLS
Several steps in a row sequence could have low but comparable rate constants ⇒ kinetically relevant not RLS/RDS.
转换频率(Turnover freq.):
\[ TOF = \frac {r}{[L]} \]
Most Abundant Reaction Intermediate (MARI):
\[ \theta_i \sim 1 \]
Chapter 5: Chemical Kinetics II RDS
let $k_{-2} =0 $
$$ \begin{aligned} \frac{d[I]}{dt} = 0 &= k_1[R] - k_{-1}[I] - k_2 [I] + k_{-2}[P] \ &= A\exp(-\frac{\Delta G_1^\ddagger}{RT}) [R] - A\exp-\frac{\Delta G_1^\ddagger+\Delta G_1}{RT} - A\exp-\frac{\Delta G_2^\ddagger}{RT}
\end{aligned} $$
when $\Delta G_1^\ddagger + \Delta G_1 \ll \Delta G_2^\ddagger $ :
\[ \begin{gathered} \exp(-\frac{\Delta G_1^\ddagger}{RT}) [R] - \exp[-\frac{\Delta G_1^\ddagger+\Delta G_1}{RT}](I) = 0 \\ [R] = \exp[-\frac{\Delta G_1}{RT}](I)\\ r = k_2[I] = A\exp(-\frac{\Delta G_2^\ddagger}{RT})\exp[\frac{\Delta G_1}{RT}](R) \\ = A\exp[-\frac{\Delta G_2^\ddagger - \Delta G_1}{RT}](R) \end{gathered} \]
Only the highest barrier determines the rate.
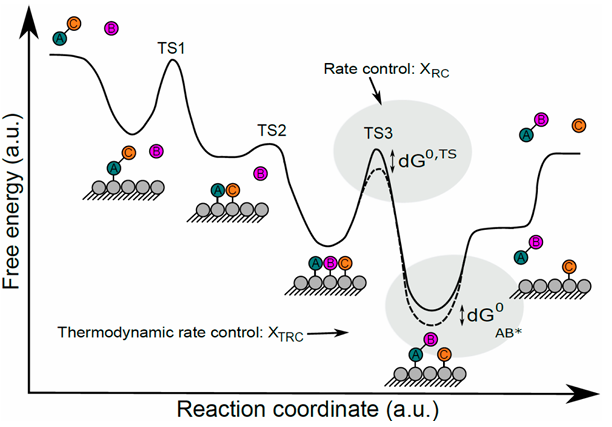
Degree of Rate Control (DRC) define the DRC of elementary step i :
\[ X_{RC,i} = \frac{k_i}{r}(\frac{\partial r}{\partial k_i})_{k_{j \neq i}, K_i} = (\frac{\partial \ln r}{\partial \ln k_i})_{k_{j \neq i}, K_i} \]
for RDS on step i :
\[ X_{RC,i} = 1, \quad X_{RC, j\neq i} = 0 \]
from TST :
$$ \begin{aligned} X_{RC,i} &= (\frac{\partial \ln r}{\partial \ln k_i}){k \ &= (\frac{\partial \ln r}{\partial \ln (\frac{k_BT}{h} \exp(\frac{-\Delta G_i^\ddagger}{RT}))})}, K_i{k \ &= (\frac{\partial \ln r}{\partial (\frac{-\Delta G_i^\ddagger}{RT})})}, K_i{k }, K_i
\end{aligned} $$
we can also define Degreee of thermodynamic controlled :
\[ \begin{aligned} X_{TC,i} &= (\frac{\partial \ln r}{\partial (\frac{-\Delta G_i}{RT})})_{k_{j \neq i}, K_i} \end{aligned} \]
for eg:
$$ \begin{gathered} \ce{A + \ast <--> A\ast} &K_1 \ \ce{B + \ast <--> B\ast} &K_2 \ \ce{A\ast + B\ast -> AB\ast + \ast} &RDS, k_3\ \ce{AB\ast <--> AB + \ast}
\end{gathered} $$
\[ K_1 = \frac{\theta_A}{P_A \cdot \theta_*} \quad K_2 = \frac{\theta_B}{P_B \cdot \theta_B} \]
\[ \theta_A + \theta_B + \theta_* = 1 \]
若A为MARI物种: [ \theta_* = \frac{1}{P_A K_1} \quad \theta_B = \frac{K_2 P_B}{K_1 P_A} ]
\[ \tau = k_3 \theta_A \theta_B = \frac{k_3 K_2}{K_1} \frac{P_B}{P_A}= \frac{k_B T}{h} \exp \left[ -\frac{\Delta^\ddagger G_3^0 + \Delta G_2^0 - \Delta G_1^0}{RT} \right] \frac{P_B}{P_A} \]
\[ = \frac{k_B T}{h} \exp \left[ -\frac{\neq G_3^0 - \Delta G_1^0 - \Delta G_2^0 + \Delta G_2^0 - \Delta G_1^0}{RT} \right] \frac{P_B}{P_A} \]
\[ = \frac{k_B T}{h} \exp \left[ -\frac{\neq G_3^0 - 2G_A^0}{RT} \right] \frac{P_B}{P_A} \]
可得\(X_{A,RC} = -2\) ,为毒化剂作用
\(X_{\ddagger,RC} = 1\) 意味着RDS控制反应速率
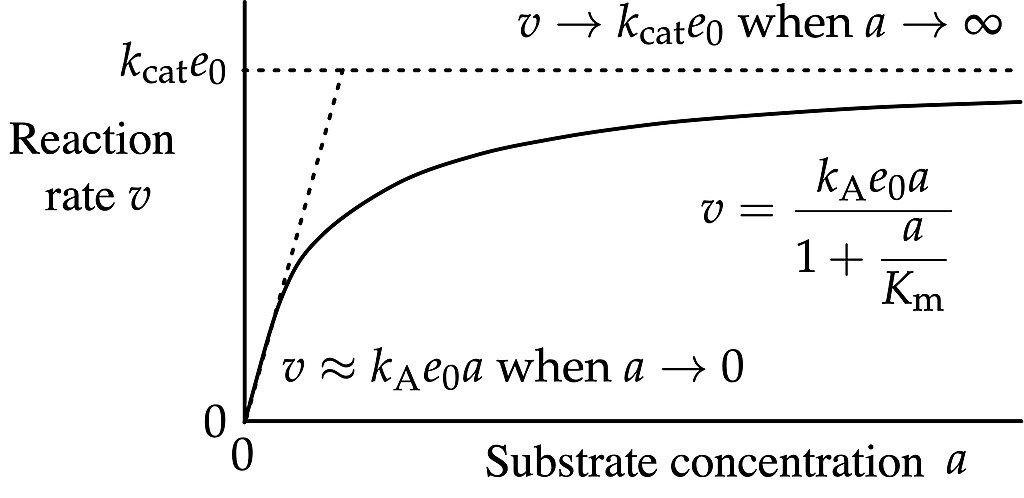
Enzymatic Catalysis Michaelis Manton kinetics
\[ r = \frac{r_{max}[S]}{K_m + [S]} \]
Reaction with 2 substates:
\[ \begin{gathered} E+S_1 \rightleftharpoons ES_1 &K_1\\ E+S_2 \rightleftharpoons ES_2 &K_2\\ ES_1+S_2 \rightleftharpoons ES_1S_2 &K_3\\ ES_2+S_1 \rightleftharpoons ES_1S_2 &K_4\\ ES_1S_2 \rightarrow E+P &K_5\\ K_1 = \frac{[E][S_1]}{[ES_1]} \quad K_2 = \frac{[E][S_2]}{[ES_2]} \\ K_3 = \frac{[ES_1][S_2]}{[ES_1S_2]} \quad K_4 = \frac{[ES_2][S_1]}{[ES_1S_2]} \end{gathered} \]
solve :
\[ r = \frac{k[E_0]}{1+\frac{k_4}{[S_0]} + \frac{k_3}{[S_2]} + \frac{k_2k_4 + k_2k_3}{[S_1][S_2]}} \]
Assuming \([S_2]\) is large :
\[ r = \frac{\frac{k[E_0][S_2]}{[S_2]+K_3} \cdot [S_1]}{\frac{K_1K_3 + K_4[S_2]}{[S_2]+K_3}+[S_1]} = \frac{k[E_0][S_1]}{K_4 + [S_1]} \]
Kinetics in thermal dynamically non-ideal systems
\[ \ce{A + B <--> X^\ddagger -> P} \]
应用艾琳方程:
\[ r = \frac{k_BT}{h}c_x^\ddagger = \frac{k_BT}{h}k^\ddagger \frac{\gamma_A \gamma_B}{\gamma^\ddagger} c_Ac_B \]
we have Broused-Bjerrum relation
\[ k = k_1\frac{\gamma_A \gamma_B}{\gamma^\ddagger} \]
Reaction is dilute electrolytes \[ \lg \frac{k}{k_0} = -cZ_A^2\sqrt{I}-cZ_B^2\sqrt{I}+c(Z_A+Z_B)^2\sqrt{I} = 2cZ_AZ_B\sqrt{I} \]
if \(Z_A\) or \(Z_B = 0\) ,\(k = k_0\)
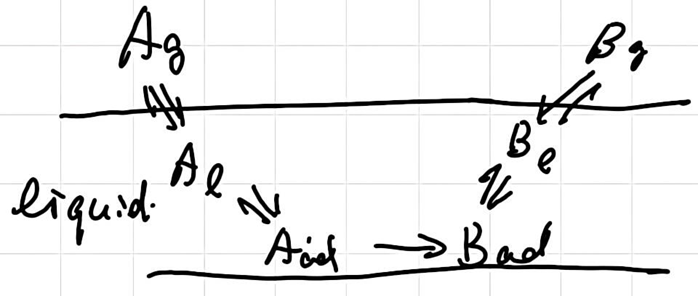
环己烯的氢气加成 on Pt:
H₂(g) ⇌ H₂(l) // 溶解
H₂(l) ⇌ H₂(p) // p: 物理吸附于表面
H₂(p) + ** → 2H* (k₃) // **RDS:氢分子解离**
\[ \begin{aligned} r &= \frac{k_BT}{h} k^\ddagger \frac{\gamma_{H2}^p}{\gamma^\ddagger} c_{H2}^p \\ &= \frac{k_BT}{h} K_Hk^\ddagger \frac{\gamma_{H2}^l}{\gamma^\ddagger} c_{H2}^l \end{aligned} \]
由于放热步骤,可认为过渡态和H2活度系数相近。于是\(r \propto K_H c^l_{H2}\) 依赖于溶剂。
on Pd:
H₂(g) ⇌ H₂(l) ⇌ H₂(p) ⇌ 2H* (快速平衡)
RH* + H* ⇌ X^\neq → RH₂** (k₆ₐ, **RDS:表面加氢**)
\[ \begin{aligned} r &= \frac{k_BT}{h} k^\ddagger \frac{\gamma_{RH*}c_{RH*}\gamma_{H*}c_{H*}}{\gamma^\ddagger} \\ &= L\frac{k_BT}{h} k^\ddagger \frac{\gamma_{RH*}}{\gamma^\ddagger}\sqrt{K_H^1p_{H2}a_{**}} \end{aligned} \]
RH为MARI物种,其物理状态受金属表面作用支配而与溶剂无关。
补偿效应 分解反应活化熵增加而活化焓也增加 缩合反应活化熵减小而活化焓也减小 焓熵相互补偿,导致\(k\) 变化不大。考虑水合:
反应器 间歇式反应器 Batch 系统类型 :封闭系统 。混合方式 :投料时瞬间混合,反应过程中无混合 。浓度特征 :所有组分的浓度随时间不断变化 。不存在真正的稳态。应用 :常用于小批量、多品种的生产,如制药、精细化工。 连续搅拌釜式反应器 Continuously stirred tank reactor 系统类型 :开放系统 。混合方式 :瞬间、全混 。进料一进入CSTR,其组成立即与釜内及出口物料完全相同。浓度特征 :釜内各处浓度均匀,且不随时间变化(稳态) ,但等于出口的低浓度 。应用 :适用于对返混要求不高的反应,易于控制温度和连续操作。 活塞流反应器 l Pug-flow reaction 系统类型 :开放系统 。混合方式 :在垂直于流动的方向上无混合 ;在沿流动的方向上无混合 。流体像“活塞”一样向前推进。浓度特征 :反应物浓度沿反应器长度方向逐渐降低 。在任一特定截面上,组成是均匀的。应用 :适用于要求高转化率的反应,因为它避免了返混导致的效率下降,是工业上最常用的反应器类型之一。 Chapter 6 Electrode Kinetics Electro Kinetics
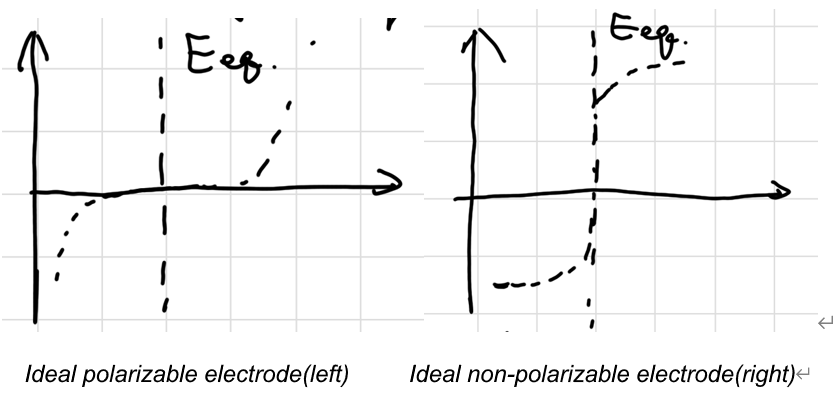
理想可极化电极:不允许电子通过,只能积累电荷,电极本身不发生电化学反应,如惰性电极
理想不可极化电极:允许电子通过,反应快且电位基本不变,如参比电极
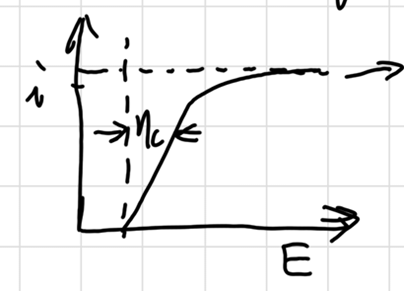
实际存在过电位:
\[ \eta = E - E_{eq} \]
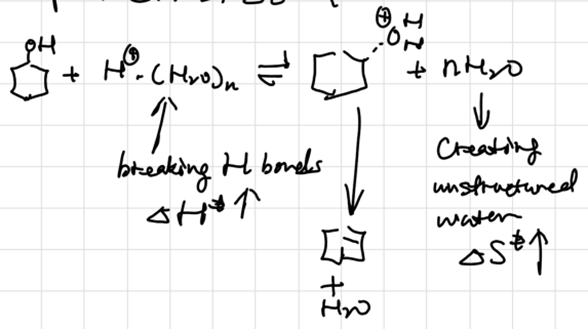
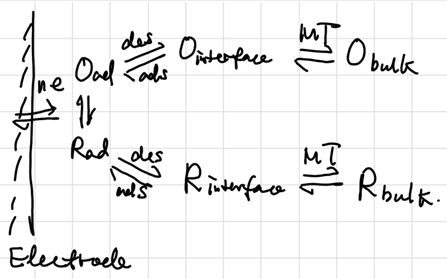
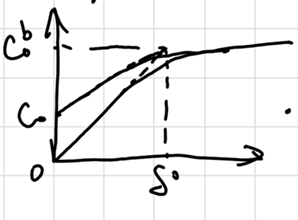
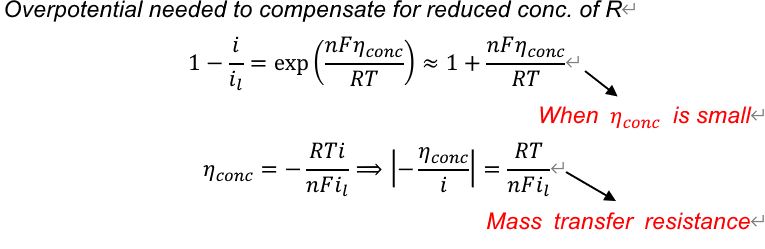
Semi-empirical Steady-State Treatment 扩散层:假定电极附近有一段无法搅拌的,厚度为\(\delta\) 的停滞层(stagnant layer ),其浓度是线性减小的:
\[ v_{mt} = D_O (\dv{C_O}{x})_{x=0} = D_O \frac{C_O^b - C_O}{\delta_O} = m_O(C_O^b - C_O) \]
where \(m = D / \delta\) stands for 质量传递系数(mass transfer coef.). 若认为单位时间的扩散通量可用电流表示:
\[ \frac{i}{nFA} = m_O(C_O^b - C_O) = m_R(C_R - C_R^b) \]
当\(C_O = 0\) 时达到极限电流:
\[ i_l = nFAm_OC_O^b, \quad \frac{C_O}{C_b} = 1-\frac{i}{i_l}, \quad E = E^{\circ\prime} + \frac{RT}{nF}\ln\frac{i_l-i}{i_l} \]
if R initially absent:
\[ \begin{gathered} C^b_R = 0, C_R = \frac{i}{nAFm_R} \\ C_O = (1-\frac{i}{i_l})\frac{i_l}{nAFm_O} \\ E = E^{\circ\prime} - \frac{RT}{nF} \ln \frac{m_O}{m_R} + \frac{RT}{nF} \ln \frac{i_l - i}{i_l} \end{gathered} \]
令半波电位\(i = i_l/2\) :
\[ E_{1/2} = E^{\circ\prime} - \frac{RT}{nF} \ln \frac{m_O}{m_R} \]
可反映相对扩散速率
id R is isolable:
\[ \begin{gathered} a_R = 1 \\ E = E^{\circ\prime} + \frac{RT}{nF}\ln C_O = E^{\circ\prime} + \frac{RT}{nF}\ln\frac{i_l-i}{i_l} + \frac{RT}{nF}\ln{C_O^b} \end{gathered} \]
when i=0, \(C_O = C_O^b\) :
\[ E_eq = E^{\circ\prime} +\frac{RT}{nF}\ln{C_O^b} \]
于是过电位:
\[ \eta = |E-E_{eq}| = |\frac{RT}{nF}\ln\frac{i_l-i}{i_l}| \]
Electrode kinetics Tafel Equation :
\[ \eta = a+b\log i \]
b:Tafel 斜率 (mV/dec),表示电流增一数量级所需的电位变化
Bulter-Volmer Kinetics
Chapter 5: Phase Diagrams Canonical ensemble 正则系综 define
\[ \alpha = -\frac{\mu}{kT}, \beta = \frac 1{kT} \]
we have mixing entropy(用概率代替浓度):
\[ S = -k\sum_I f_i\ln f_i \]
and two condtrains:
\[ \begin{cases} \sum_i f_i = 1 \\ \sum_i f_i E_i = U \end{cases} \]
when reaching maximum entropy, we can derive:
\[ f_i = \frac{e^{-\beta E_i}}{\sum_ie^{-\beta E_i}} = \frac{e^{-\beta E_i}}{Z(V,T,N)} \]
where \(Z(V,T,N)\) 是正则配分函数
在这里用离散和是不精确的,我们改成积分形式。by using H, for single partical:
\[ Z = \frac{1}{h^3} \int e^{-\beta H(\vec x,\vec p)} d^3\vec x d^3\vec p \]
and for many partials:
\[ Z = \frac{1}{h^{3N}} \int \prod_{i=1}^{N} e^{-\beta H(\vec x_i , \vec p_i)} d^{3}\vec x_i d^{3} \vec p_i \]
if particals are 全同:
\[ Z = \frac{1}{N!h^{3N}} \int \prod_{i=1}^{N} e^{-\beta H(\vec x_i , \vec p_i)} d^{3}\vec x_i d^{3} \vec p_i = \frac{1}{N!h^{3N}}\pqty{\int \prod_{i=1}^{N} e^{-\beta U(\vec x_i)} d^{3}\vec x_i}\pqty{\int \prod_{i=1}^{N} e^{-\beta p^2/2m} d^{3}\vec p_i} \]
这些即认为最小的单位空间是\(\frac 1{h^3}\) ,把N个粒子分配到这些空间里。
通过配分函数计算能量的期望值:
\[ \begin{aligned} \left< Z \right> = \sum_i E_iP_i &= \frac1Z \sum_i E_ie^{-\beta E_i}\\ &= -\frac1Z \frac{\partial}{\partial\beta} Z(\beta, E_i) \\ &= -\frac{\partial \ln Z}{\partial \beta} = kT^2\frac{\partial \ln Z}{\partial T} \end{aligned} \]
进而还可得出自由能的关系,我们有:
\[ \begin{gathered} S = -k\sum f_i \ln f_i = -\frac kZ\sum (-\beta E_i - \ln Z)e^{-\beta E_i} = k(\ln Z + \beta\left< E \right>) \\ \left< E \right> = U \\ F = U-TS = -kT\ln Z \end{gathered} \]
巨配分函数\(\Xi\) :
\[ \Xi(\mu, V,T) = \sum_{N=0}^\infty Z(N,V,T) e^{N\beta\mu} = 1+\sum_{N=1}^\infty Z(N,V,T) \lambda^N \]
where \(\lambda = e^{\beta\mu}\) represent absolute acivity(绝对活度)
回到气体,对单个粒子,由于动能项积分可以从麦克斯韦分布推导,我们有:
\[ Z_N = \frac1{N!h^{3N}} \pqty{2\pi mkT}^{3N/2} \int e^{-\beta U(\vec x)} d^3\vec x \]
定义构型积分:
\[ Q_N = \int e^{\sum_{i<j} U(r_{ij})} \dd r_1 ... \dd r_N \]
于是:
\[ Z_N = \frac1{N!} \pqty{\frac{2\pi mkT}{h^2}}^{3N/2} Q_N \]
于是我们有:
\[ \Xi = \sum_{N=0}^{\infty} \frac{z^N}{N!}Q_N \qc z = \lambda \pqty{\frac{2\pi mkT}{h^2}}^{3/2} \]
压强在恒体积下:
\[ P = kT\pqty{\pdv{\ln \Xi}{V}}_{T, \mu} = \frac{kT}{\Xi}\sum_{N=0}^{\infty} \frac{z^N}{N!} \pdv{Z_N}{V} = \frac{kT}{\Xi V}\sum_{N=0}^{\infty} \frac{z^N}{N!} Z_N \]
这里我们引入集团展开 (Cluster Expansion)。我们知道维利展开:
\[ \frac{P}{kT} = \rho + B_2(T) \rho^2 + B_3(T)\rho^3 ... = b_1 z + b_2z^2 + b_3z^3 ... \]
两边系数对比:
\[ (\sum_{j=1}^{\infty} b_j z^j ) = \frac{1}{V}\ln(1 + \sum_{N=1}^\infty \frac{z^N}{N!} Q_N) \]
事实上,在这里泰勒硬展开就可以得到 \(b_j\) 的各个解,但这样太麻烦了。不过比较第一项就可以得到:
\[ b_1 = Q_1/V = 1 \\ b_2 = -\frac{1}{2V}(Z_2 - Z_1)^2 \]
这是由于此时没有任何外场。
引入Mayer函数 :
\[ f_{ij} = e^{-\beta U(r_{ij})} - 1 \]
于是对于稀薄气体展开:
\[ \prod_{i<j} e^{-\beta U(r_{ij})} = \prod_{i<j}(1+f_{ij}) = 1+\sum_{i<j} f_{ij} + \sum_{i<j,k<l} f_{ij} f_{kl} \]
\[ \rho = \ev{N}/V = \frac{kT}{V} \pqty{\pdv{\ln \Xi}{\mu}}_T = \frac{\lambda}{V}\pqty{\pdv{\ln \Xi}{\lambda}}_T = \frac{z}{V}\pqty{\pdv{\ln \Xi}{z}}_T \]
\[ \rho = \frac{z}{kT} \pqty{\pdv{P}{z}}_{V,T} = \sum_{j=1}^\infty jb_iz^{j} \]
现在假设我们认为:
\[ z = a_1 \rho + a_2 \rho^2 + a_3 \rho^3 ... \\ \rho = \sum_{j=1}^\infty jb_j(a_1 \rho + a_2 \rho^2 + a_3 \rho^3 ...)^j \]
对照上面的式子:
\[ a_1 = 1/b_1 = 1 \\ b_1a_2 + 2b_1a_1^2 = 0 \Rightarrow a_2 = -2b_2 \\ b_1a_3 + 4b_2a_1a_2 + 3b_3a_1^3 = 0 \Rightarrow a_3 = -3b_3 + 8b_2^2 \]
回到:
\[ \frac{P}{kT} = \rho + B_2(T) \rho^2 + B_3(T)\rho^3 ... = b_1 z + b_2z^2 + b_3z^3 ... \]
于是:
\[ B_2(T) = -b_2 = -\frac{1}{2V} (Q_2 - Q_1^2) \\ B_3(T) = 4b_2^2 - 3b_3 = -\frac{1}{3V^2}[V(Q_3 - 3Q_2Q_1 + 2Q_1^3) - 3(Q_2 - Q_1^2)^2] \]
再努努力!
\[ \begin{aligned} B_2(T) &= -\frac{1}{2V} \iint(e^{-\beta U(\vec r_{12})} - 1)\dd(\vec r_1)\dd(\vec r_2) \\ &= -\frac{1}{2V} \int \dd(\vec r_1) \int(e^{-\beta U(\vec r_{12})}-1)\dd(\vec r_{12}) \\ &= -\frac12 \int \dd(\vec r_1) \int(e^{-\beta U(r_{12})}-1)4\pi r_{12}^2 \dd(r_{12}) \\ &= -2\pi \int(e^{-\beta U(r)}-1)r^2 \dd r = -2\pi \int_0^\infty f_{12}r^2\dd r \end{aligned} \]
现在我们考虑 \(Q_3\) ,这时候就要考虑三体作用了。
\[ U(r_1,r_2,r_3) \simeq U(r_1,r_2) + U(r_2,r_3) + U(r_1,r_3) \]
于是:
\[ \begin{aligned} Q_3 &= \iiint e^{U_3/kT} \dd{\vec r_1} \dd{\vec r_2} \dd{\vec r_3} = \iiint(1+f_{12})(1+f_{23})(1+f_{13})\dd{\vec r_1} \dd{\vec r_2} \dd{\vec r_3} \\ &= \iiint(f_{12}f_{23}f_{13} + f_{12}f_{23} + f_{12}f_{13} + f_{23}f_{13} + f_{12} + f_{13} + f_{23} + 1)\dd{\vec r_1} \dd{\vec r_2} \dd{\vec r_3} \end{aligned} \]
经过一堆化简可以得到:
\[ B_3(T) = -\frac{1}{3V}\iiint f_{12}f_{23}f_{13}\dd{\vec r_1} \dd{\vec r_2} \dd{\vec r_3} = -\frac{1}{3V}\iiint \triangle \dd{\vec r_1} \dd{\vec r_2} \dd{\vec r_3} \]
其中 \(\triangle\) 表示这是三个粒子两两作用的结果
对于第四位力系数:
\[ B_4(T) = \iiiint (3\square + 6\oslash + 1\boxtimes) \dd{\vec r_1} \dd{\vec r_2} \dd{\vec r_3} \dd{\vec r_4} \]
其中对于四粒子系统,有3种 \(\square\) 作用,6种 \(\oslash\) 作用,1种 \(\boxtimes\) 作用。
for 维利展开:
\[ B_2 = -2\pi \int_0^\infty [e^{-\beta u(r)} - 1] r^2 \dd r \]
hard-sphere :
\[ u(r) = \begin{cases} \infty ,&r<\sigma \\ 0 ,&r\ge \sigma \end{cases} \]
\[ B_2 = -2\pi \int_0^\sigma (-1)r^2\dd r = \frac{2\pi}{3} \]
square-well potential :
\[ u(r) = \begin{cases} \infty &r<\sigma \\ -\epsilon &\sigma<r<\lambda\sigma \\ 0 &r>\sigma \end{cases} \]
\[ B_2(T) = b_0{(1-(\lambda^3-1)(e^{\beta\epsilon}-1))} \]
Lennard-Jones potential :
\[ u(r) = 4\epsilon [(\sigma/r)^{12} - (\sigma/r)^6] \]
\[ B_2 = \frac 1Z \int_0^\infty( e^{-\beta u(r)}-1) 4\pi r^2 \dd r \]
Chapter 6 Liquid cases: simple liquid theory for 傻逼 case, He liquid 不考虑量子效应,直观上看就是解一个原子和周围的核和电子的关系,这里就要用B-O近似。
一个粒子包含六个信息:
\[ N(x_1, y_1,z_1,p_x,p_y,p_z) \]
将N个位置出现粒子的概率用向量表示:
\[ P^{(N)} (\vec r_1 , \vec r_2, \vec r_3, ...\vec r_N, \vec p_1, \vec p_2, \vec p_3,...\vec p_N) \]
现在我们假设只关心n个粒子的位置状态,其他的通过积分平均掉:
\[ P^{(n)} (\vec r_1 , \vec r_2, \vec r_3, ...\vec r_n) {\dd}\vec r_n ... {\dd}\vec r_1 = \int...\int P^{(n)} (\vec r_1 , \vec r_2, \vec r_3, ...\vec r_n) {\dd}\vec r_n ... {\dd}\vec r_1{\dd}r_{n+1} ... \dd r_N \]
消掉两边
\[ P^{(n)} (\vec r_1 , \vec r_2, \vec r_3, ...\vec r_n) = \int...\int P^{(n)} (\vec r_1 , \vec r_2, \vec r_3, ...\vec r_n) {\dd}r_{n+1} ... \dd r_N = \int ... \int \frac{e^{-\beta U_N}}{Z_N} {\dd}r_{n+1} ... \dd r_N \]
密度分布函数:在给定位置\(r_1, ...,r_n\) 同时找到n个粒子的数密度
\[ \rho^{(n)}(\vec r_1 , \vec r_2, \vec r_3, ...\vec r_n) = \frac{N!}{(N-n)!(n)!}P^{(N)} \]
\[ \rho^{(1)}(\vec r_1 , \vec r_2, \vec r_3, ...\vec r_n) = \frac{N!}{(N-1)!}P^{(N)} = N\int ... \int \frac{e^{-\beta U_N}}{Z_N} {\dd}r_2 ... \dd r_N = N/V \]
对均匀体系,显然找到一个粒子的密度分布是是密度常数 \(\rho\)
\[ \int \rho \dd r = N \qc \rho V = N \]
correlation function n粒子相关函数 (找到粒子\(r\) 的相对概率):
\[ g^{(n)} = \frac{\rho^{(n)}(\vec r_1 , \vec r_2, \vec r_3, ...\vec r_n)}{\rho^n} \]
\[ g^{(2)} = \frac{V^2}{N^2} \frac{N(N-1)}{2}\int ... \int \frac{e^{-\beta U_N}}{Z_N} {\dd}r_{3} ... \dd r_N \]
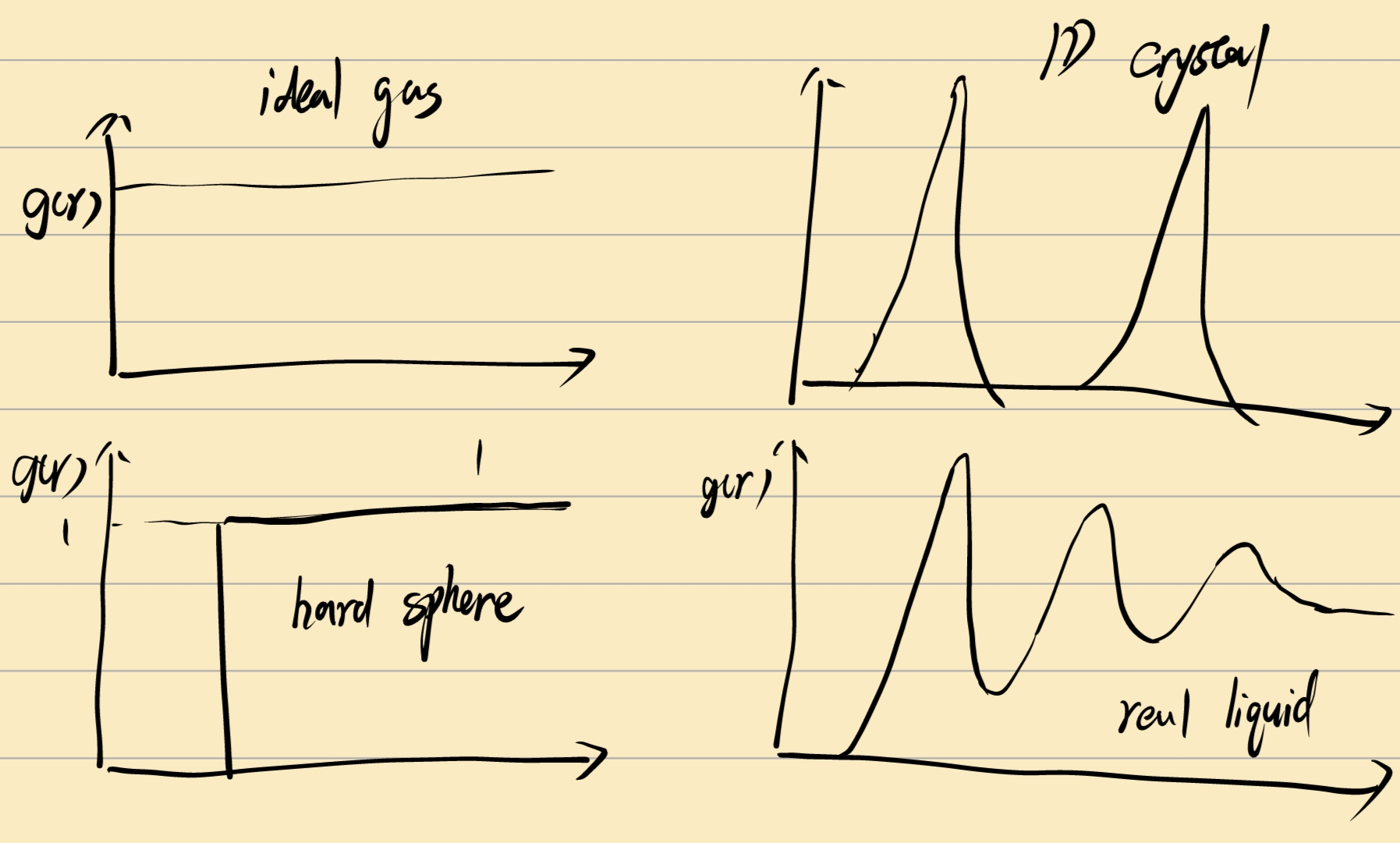
for sphere-symmetry molecules, \(g^{(2)}\) only depend on \((r_1 - r_2)\)
对这里的 \(g(r)\) 而言,归一化积分,得到的应该是除了参考粒子外得到所有其他粒子数 \(N-1\) :
\[ \int_0^\infty \rho g(r)4\pi r^2\dd r = N-1 \]
对于相关函数:
这里液体呈现出类似1D-crystal的关系,这是由于液体由于熵效应,在半径\(\sigma\) 的地方出现粒子的概率很大,相应的在中间出现的概率很小。
引入结构因子 Structrural factor:
\[ h(r) = g(r)-1 \]
for pair-wise additive potential :
\[ U_N(r_1, r_2,r_3...r_N) = \sum_{i<j} U(r_{ij}) = \frac{N(N-1)}{2}U_{12} \]
\[ \overline E = kT^2\pqty{\pdv{\ln Q}{T}} = 3/2 NkT + \overline U \]
\[ \overline{U} = \frac{\int...\int e^{-\beta U_N}\dd r_1 ... \dd r_N}{Z_N} = \frac{N(N-1)}{2} \frac{\int...\int e^{-\beta U_N}\dd r_1 ... \dd r_N}{Z_N} \]
经过一番推导:
\[ \overline U = \frac{4\pi N^2}{V} \int_0^\infty U(r)g(r) r^2 \dd r \]
so \(\overline E = ...\)
\[ P = kT(\pdv{\ln Q}{V})_{N,T} = kT(\pdv{\ln Z_N}{V})_{N,T} \]
where
\[ Z_N = \int_0^{V^{1/3}} \int_0^{V^{1/3}} \int_0^{V^{1/3}} e^{-\beta U_N} \dd x_1 \dd y_1 \dd z_1 ... \dd x_n \dd y_n \dd z_n \]
作变量替换
\[ x_i' = x_i / V^{1/3} \]
\[ Z_N = \int_0^{1} \int_0^{1} \int_0^{1} e^{-\beta U(x_1'V^{1/3}...)} \dd x_1' \dd y_1' \dd z_1' ... \dd x_n' \dd y_n' \dd z_n' \]
于是
\[ \begin{aligned} \pdv{Z}{V} &= NV^{N-1} \int_0^{1} \int_0^{1} \int_0^{1} e^{-\beta U_N} \dd x_1' \dd y_1' \dd z_1' ... \dd x_n' \dd y_n' \dd z_n' \\ & \quad -V^N\int_0^{1} \int_0^{1} \int_0^{1} \beta e^{-\beta U_N} \pdv{U_N}{V} \dd x_1' \dd y_1' \dd z_1' ... \dd x_n' \dd y_n' \dd z_n' \end{aligned} \]
解构一下:
\[ \pdv{U_N}{V} = \sum_{i<j} \dv{U_{ij}}{{r_{ij}}}\dv{{r_{ij}}}{V} = \sum_{i<j} \dv{U_{ij}}{{r_{ij}}} (\frac13 V^{-\frac23}r_{ij}') \]
\[ \pdv{\ln Z}{V} = \rho - \frac{\rho^2}{3VkT}\int...\int r_{12} \dv{U(r_{r_12})}{r_{12}} g^{(2)}(r_1,r_2) \dd r_1 \dd r_2 \]
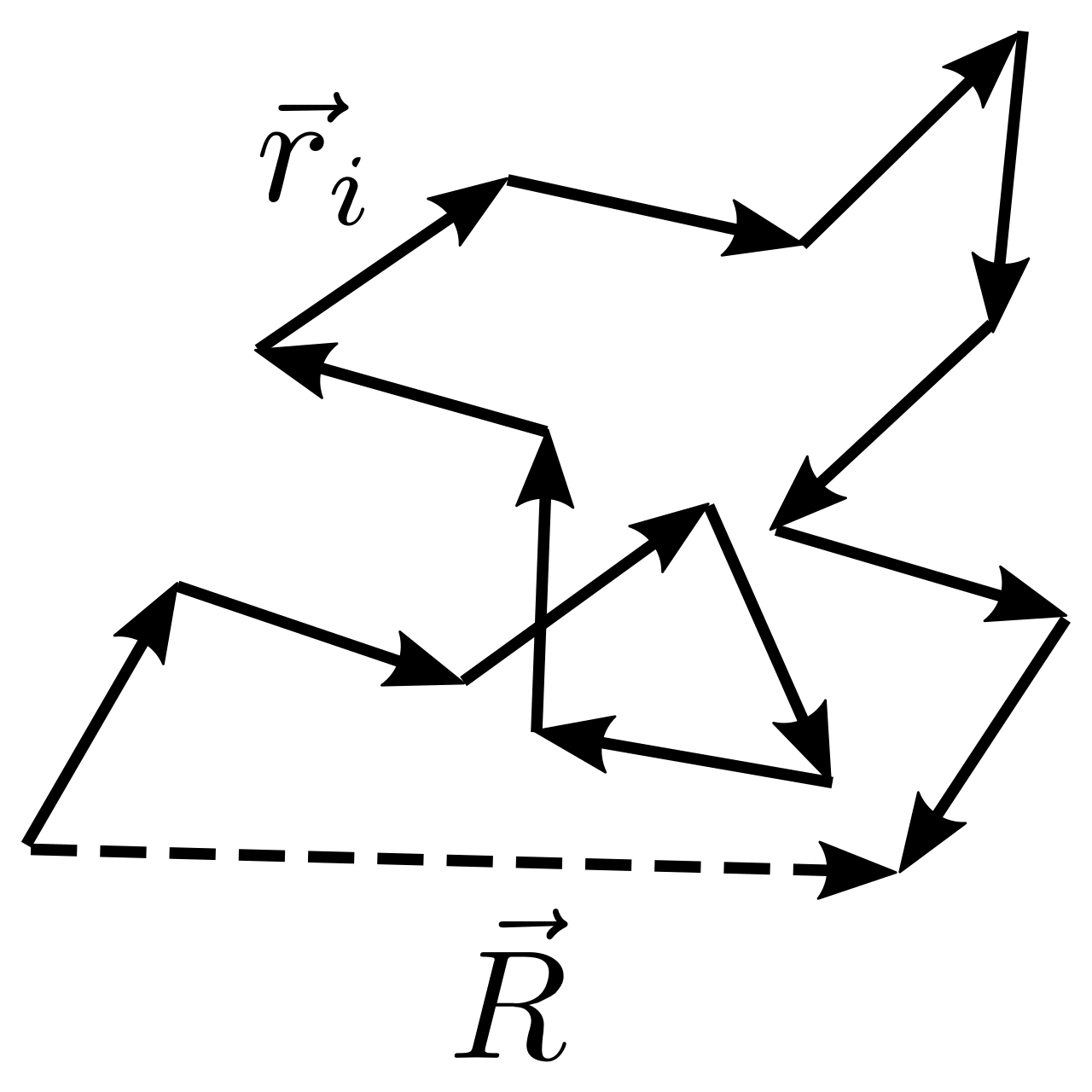
Chapter 7 Polymer different levels primary structure secondary structure tertiary structure quantinary structure simple example: random coil for freely joint chain:
\(N\) as number of residues, \(l\) as monomer length, \(nl\) as end-to-end length.
define \(R_c = Nl\) as counter length:
\[ N_L + N_R = N \\ n = N_L - N_R \]
so:
\[ W = \frac{N!}{N_L!N_R!} = \frac{N!}{(\frac{N+n}{2})!(\frac{N-n}{2})!} \]
probability of observing on end-to-end distance of \(nl\) is:
\[ P = \frac{W}{2^N} = \frac{N!}{2^N(\frac{N+n}{2})!(\frac{N-n}{2})!} \]
so
\[ \ln P \approx \sqrt{\frac2{\pi N}} - \frac12 N(\frac{n}{N})^2 \]
for 1D cases:
\[ P = \sqrt{\frac2{\pi N}}e^{-\frac{h^2}{2N}} \]
for 3D cases:
\[ f =4\pi\pqty{\frac{3}{2\pi Nl^2}}^{3/2} R^2e^{-\frac{3R^2}{2Nl^2}} \]
so RMSD
\[ RMSD = \sqrt{\int f(R)R^2dR} \]
conformation entropy:
\[ S = k\ln W \]
and we know:
\[ S_{max} = S(n=0) \]
def \(\nu = n/N\) , we got
\[ \Delta S = -\frac12 kN \ln\Bqty{(1+\nu)^{1+\nu} \cdot (1-\nu)^{1-\nu}} \]
calculation of chemical potential Free energy:
\[ -F = kT\ln Q = \ln Z_N - \ln N! - 3N\ln\Lambda \]
and
\[ \mu = \pdv{F}{N} = F(N,V,T) - F(N-1,V,T) \]
\[ \mu = -kT\bqty{\ln(Z_N/Z_{N-1}) - \ln N - \ln \Lambda^3} \]
这里我们需要计算一个比较难算的量 构型积分之比。我们引入一个参数 \(\xi\) 称为耦合参数,现在我们假设第 \(N\) 个粒子是一个
so
\[ Z_N(\xi) = \iint e^{-\frac{U(\xi)}{kT}} \dd r_i \dd r_N \]
where
\[ U_N(r_1, \ldots r_N, \xi) = \sum_{j=2}^N \xi U(r_{1j}) + \sum_{2\le i,j \le N} U(r_{ij}) \]
\[ Z_N(\xi = 0) = \iint e^{-U(r_2,r_N)/kT}\dd r_2 \dd r_N \int 1\dd r_1 = Z_{N-1}V \]
so 原始转化为
\[ \ln (Z_N/Z_{N-1}) = \ln(Z_N(\xi=1)/Z_N(\xi = 0)) + \ln V = \ln V + \int_0^1 \pdv{\ln Z_N}{\xi} d\xi \]
\[ \pdv{Z_N}{\xi} = -\beta \int\cdots\int e^{-\beta U(\xi)} \bqty{\sum_{j=2}^N U_{ij}} \dd r_1 \cdots \dd r_N \]
\[ \begin{aligned} \pdv{\ln Z_N}{\xi} &= -\beta \int\cdots\int \frac{e^{-\beta U(\xi)}}{Z_N} \bqty{\sum_{j=2}^N U_{ij}} \dd r_1 \cdots \dd r_N \\ &= -\beta \int\cdots\int \frac{(N-1)e^{-\beta U(\xi)}}{Z_N}\dd r_3 \cdots \dd r_N \ U_{12} \dd r_1 \dd r_2 \\ &= -4\pi\beta\rho \int_0^\infty U(r)g(r,\xi,T) r^2 \dd r \end{aligned} \]
于是就可以表达出化学势。
perturbation free energy calculation \[ \begin{aligned} \Delta F &= F_y - F_x \\ &= -kT \ln\frac{\iint e^{-\beta H_y}\dd r \dd p}{\iint e^{-\beta H_x}\dd r \dd p} \\ &= -kT \ln\frac{\iint e^{-\beta (H_y-H_x)}e^{-\beta H_x}\dd r \dd p}{\iint e^{-\beta H_x}\dd r \dd p} \\ &= -kT\ev{e^{-\beta(H_y - H_x)}}_x \end{aligned} \]
然而这样很多情况会失败,原因在于据大多数情况,对于偏离平衡态的 \(H_y\) 能量会特别高,导致这个数特别小甚至就是0.
为了避免这一点,我们定义一个耦合参数 \(\lambda\) 进行插值:
\[ H(\lambda) = (1-\lambda)H_x + \lambda H_y \]
这样我们就可以积分计算:
\[ \begin{aligned} \pdv{F(\lambda)}{\lambda} &= -kT\frac1Q\pdv{Q}{\lambda} \\ &= \frac1Q \iint \pdv{H_\lambda}{\lambda}e^{-\beta H_\lambda} \dd r \dd p \\ &= \ev{\pdv{H(\lambda)}{\lambda}}_\lambda \end{aligned} \]
于是
\[ \Delta F = F_y - F_x = \int_0^1 \ev{H_y - H_x}_\lambda \dd \lambda \]
在实际计算中,通常 \(P(F) = e^{-\beta H}/Q\) 是一个突跃值,实际上我i们可以通过测量及其多个温度下的曲线并作其抛物线:
\[ P(H) = \sum_i n_i e^{-\beta_iH} \]
Chapter 8 Brownian motion Lagevin Equation assume a motion satisfied Lagevin Equation :
\[ \dv{u}{t} = -\zeta u + A(t) \]
where \(A(t)\) stands a random force.
assume \(u = c(t)e^{-\zeta t}\) :
\[ \begin{gathered} \dv{c}{t} e^{-\zeta t} - \zeta ce^{-\zeta t} = -\zeta u + A(t) \\ \dv{c}{t} = A(t)e^{\zeta t} \end{gathered} \]
integrate:
\[ c = c_0 + \int_0^t A(t') e^{\zeta t'}\dd t' \]
transform into u:
\[ u = u_0e^{-\zeta t} + e^{-\zeta t}\int_0^t A(t') e^{\zeta t'}\dd t' \]
and we can know a random value's average equals to zero:
\[ \ev{u} = u_0 e^{-\zeta t} \]
for the RMSE:
\[ \begin{aligned} \ev{(u-\ev u^2)} &= \ev{u^2} - 2\ev u^2 + \ev u^2 \\ &= \ev{u^2} - \ev{u}^2 \end{aligned} \]
for derectly case:
\[ \begin{aligned} \ev{(u-\ev u^2)} &= \ev{\bqty{e^{\zeta t' } \int_0^t e^{\zeta t } A(t') \dd t'}^2} \\ &= \ev{e^{2\zeta t } \int_0^t\int_0^t e^{\zeta t' } A(t') e^{\zeta t^{\prime\prime}} A(t^{\prime\prime}) \dd t'\dd t^{\prime\prime}} \\ &= e^{2\zeta t } \int_0^t\int_0^t e^{\zeta t'} e^{\zeta t^{\prime\prime}} \ev{ A(t')A(t^{\prime\prime})} \dd t'\dd t^{\prime\prime} \end{aligned} \]
what the fucking is \(\ev{ A(t')A(t^{\prime\prime})}\) ?
let \(\ev{ A(t')A(t^{\prime\prime})} = \ev{A(0)A(t^{\prime\prime} - A')} = \phi(t^{\prime\prime}-t')\) , this forces is prop to delta function因为作用时间极短:
\[ \phi(t^{\prime\prime}-t') = \Gamma\delta(t^{\prime\prime}-t') \]
\[ \begin{aligned} \ev{(u-\ev u^2)} &= e^{2\zeta t } \int_0^t\int_0^t e^{\zeta (t' + t^{\prime\prime})} \phi(t^{\prime\prime}-t') \dd t'\dd t^{\prime\prime} \\ &= e^{2\zeta t } \int_0^t\int_0^t e^{\zeta \tau'} \phi(\tau^{\prime\prime}) \dd (\frac{\tau' + \tau^{\prime\prime}}2 )\dd (\frac{\tau' - \tau^{\prime\prime}}2 ) \\ &= \frac 12 e^{2\zeta t } \int_0^{2t} e^{\zeta \tau'} \dd \tau' \int_{-t}^t \phi(\tau^{\prime\prime}) \dd \tau^{\prime\prime} \\ &= \frac{\tau}{2\zeta} \pqty{1-e^{-2\zeta t}} \end{aligned} \]
as \(t \to \infty\) :
\[ \ev{(\Delta u )^2} = \frac{\Gamma}{2\zeta} \]
with = 0 :
\[ \frac{m\ev{u^2}}{2} = \frac32 kT \]
that is:
\[ \frac{\Gamma}{2\zeta} = \frac{3kT}{m} \]
Generalized Langevin Eqn \[ \dv{u}{t} = \int_{-\infty}^t -\zeta(t-t') u \dd t' + A(t) \]
gotcha:
\[ \begin{aligned} r(t) - r_0 &= \int_0^t u(t') \dd t' \\ r - r_0 &= \int_0^t \bqty{u_0e^{-\zeta t'} + e^{-\zeta t'} \int_0^{t'}e^{\zeta t^{\prime\prime}} A(t^{\prime\prime}) \dd t^{\prime\prime} }dt' \\ &= \frac{1}{\zeta}u_0(1-e^{-\zeta t}) + \int_0^t\int_0^{t'} e^{-\zeta t'}e^{\zeta t^{\prime\prime}}A(t'') \dd{t''}\dd t' \\ \ev{r-r_0} &= \frac{1}{\zeta}u_0(1-e^{-\zeta t}) \end{aligned} \]
taking fucking care of RMSE:
\[ \begin{aligned} \ev{(r-r_0)^2} &= \ev{\pqty{\frac{1}{\zeta}u_0(1-e^{-\zeta t}) + \int_0^te^{-\zeta t'}\dd t'\int_0^{t'} e^{\zeta t^{\prime\prime}}A(t'') \dd{t''}}^2 } \end{aligned} \]
the later:
\[ \begin{aligned} &\int_0^te^{-\zeta t'}\dd t'\int_0^{t'} e^{\zeta t^{\prime\prime}}A(t'') \dd{t''} \\ &= -\frac{1}{\zeta}\bqty{e^{-\zeta t'}\eval{\int_0^{t'} e^{\zeta t^{\prime\prime}}A(t'') \dd{t''}}_0^t - \int_0^t e^{-\zeta t'} \dd(\int_0^{t'} e^{\zeta t^{\prime\prime}}A(t'') \dd{t''})} \\ &= -\frac{1}{\zeta}\bqty{e^{-\zeta t'}\eval{\int_0^{t'} e^{\zeta t^{\prime\prime}}A(t'') \dd{t''}}_0^t - \int_0^t A(t') \dd{t'}} \\ &= \frac{1}{\zeta}\bqty{\int_0^t \pqty{A(t') - A(t')e^{\zeta(t'-t)}}\dd t'} \end{aligned} \]
dont shock mother fucker:
\[ \begin{aligned} \ev{(r-r_0)^2} &= \pqty{\frac{1}{\zeta}u_0(1-e^{-\zeta t})}^2 + \int_0^t\int_0^t (1-e^{\zeta (t'-t)})(1- e^{\zeta (t^{\prime\prime}-t)}) \ev{ A(t')A(t^{\prime\prime})} \dd t'\dd t^{\prime\prime}\\ &= \pqty{\frac{1}{\zeta}u_0(1-e^{-\zeta t})}^2 + \frac{3kT}{m\zeta^2}(2\zeta t - 3 + 4e^{-\zeta t} - e^{-2\zeta t}) \end{aligned} \]
as \(t \to 0\) :
\[ \ev{(r-r_0)^2} = \frac{6kT}{m\zeta}t \]
with \(D = kT/m\zeta\) :
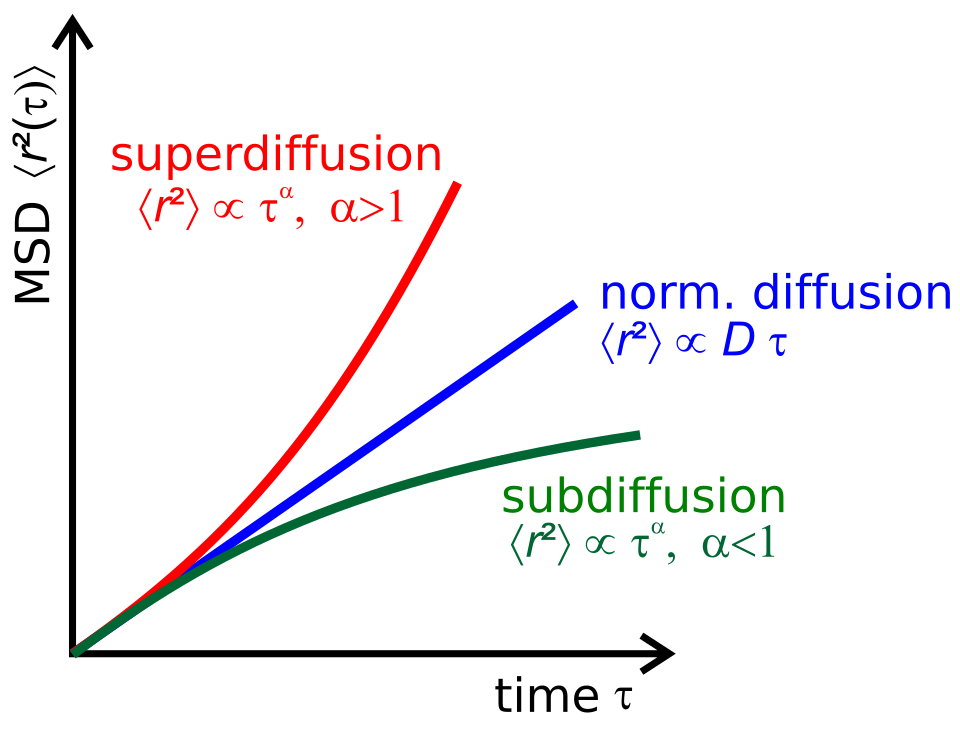
\[ \ev{(r-r_0)^2} = 6Dt \]
\[ \begin{aligned} \ev{(r-r_0)^2} &= \ev{\int_0^t u(t')\dd t' \int_0^t u(t'')\dd t''} \\ &= \int_0^t\int_0^t \ev{u(t')u(t'')} \dd t' \dd t'' \end{aligned} \]
this called velocity-velocity correlation function
\[ \begin{aligned} \ev{(r-r_0)^2} &= \int_0^t\int_0^t \ev{u(0)u(t''-t')} \dd t' \dd t'' \\ &= 2\int_0^{t-\tau}\dd t'\int_0^t \ev{u(0)u(t''-t')} \dd \tau \\ &= 2t\int_0^{t} (1-\frac{\tau}{t})\ev{u(0)u(t''-t')} \dd \tau \end{aligned} \]
going fucking \(t \to \infty\) :
\[ \ev{(r-r_0)^2} = 2t\int_0^\infty\ev{u(0)u(t''-t')} \dd \tau = 6Dt \]
Chapter 9 diffusion theorem for a certain force:
\[ m\dv{u}{t} = F - \eta u + A(t) \]
we define flux (通量)\(j_D\) :
\[ j_D = -D_x\pdv{\rho}{x} \]
where \(\rho\) indicates density distribution function. 为了简便我们先把 \(D_x\) 当常量
\[ \begin{aligned} \pdv{\rho}{t}\dd x \dd y \dd z &= j(x)\dd y \dd z - j(x+\dd x)\dd y \dd z \\ \pdv{\rho}{t}\dd x &= j(x) - \pqty{j(x) + \pdv{j}{x}\dd x} \\ \pdv{\rho}{t} &= -\pdv{j}{x} \end{aligned} \]
于是
\[ \pdv{\rho}{t} = D\pdv x\pqty{\pdv{\rho}{x}} = D\pdv[2]{\rho}{x} \]
that is called Diffusion Equation .
when \(\dv{u}{t} = 0\) ,
\[ \ev{u} = \frac{F}{m\zeta} \]
so
\[ j = j_D + j_F = \frac{\rho F}{m\zeta} - D\pdv{\rho}{x} \]
so
\[ \begin{aligned} \pdv{\rho}{t} &= -\pdv{j}{x}\\ &= \pdv{x}\pqty{\frac{\rho}{m\zeta}\pdv{V}{x}} + D\pdv[2]{\rho}{x} \end{aligned} \]
as \(t \to \infty\)
\[ \rho_{eq} = \rho_0 e^{-V(x)/k_BT} \]
Harmonic analysis \[ \dv{U}{t} = -\zeta U + A(t) \]
傅里叶展开
\[ A(t) = \sum a_\omega \cos\omega t \]
\[ a(\omega) = \frac{1}{2\pi}\int_{-\infty}^{\infty} A(t)e^{i\omega t} \dd t \]
\[ A(t) = \int_{-\infty}^{\infty} a(\omega)e^{i\omega t} \dd\omega \]
and we have
\[ a(0) = \frac1{2\pi}\int_{-\infty}^{\infty}A(t)\dd t = \frac{T\ev{A}}{2\pi} \]
for the spectrum
...
stationary
\[ \begin{aligned} I'(\omega) &= \frac{1}{{4\pi^2}}\int_{-\infty}^\infty\int_{-\infty}^\infty \ev{A(\tau)A(0)} e^{i\omega t} d\tau dt' \\ &= \frac{T}{4\pi^2}\int_{-\infty}^\infty \phi(\tau)\dd \tau \end{aligned} \]
so
\[ \begin{aligned} \mathcal F(\dv{u}{t}) &= \mathcal F[\zeta u + A(t)] \\ &= \frac{1}{2\pi} \int_{-\infty}^\infty \dv{u}{t} e^{-i\omega t} \dd t \\ &= \eval{\frac{1}{2\pi} u e^{-i\omega t}}_{-\infty}^\infty - \frac{1}{2\pi} \int_{-\infty}^{\infty}u(-i\omega)e^{-i\omega t} \dd t \\ &= i\omega\tilde{u} = \zeta\tilde u + a(\omega) \end{aligned} \]
we got
\[ \tilde u = \frac{a}{i\omega + \zeta}, \abs{\tilde u}^2 = \frac{a^2}{\omega^2 + \zeta^2} \]
and
\[ u(t) = \int \frac{a}{i\omega + \zeta} e^{i\omega t}\dd \omega \]
\[ \ev{u(t)u(0)} = \int \frac{a^2}{\omega^2 + \zeta^2}e^{i\omega t} \dd \omega = \frac{\pi a^2}{\zeta} e^{-\zeta\abs{t}} \]
Fokker-Plank Equation to calc \(\rho(u, t + \Delta t)\) given \(\rho(u-\Delta u, t)\)
\[ \rho(u, t + \Delta t) = \int \rho(u-\Delta u, t) \Psi(u-\Delta u; \Delta u) \dd(\Delta u) \]
Taylor expansion
\[ \begin{gathered} \rho(u, t + \Delta t) = \rho(u,t) + \pdv{\rho}{t} \Delta t + (\Delta t)^2 + ...\\ \rho(u-\Delta u, t) = \rho(u,t) - \pdv{\rho}{u} \Delta u +...\\ \Psi(u-\Delta u; \Delta u) = \Psi(u; \Delta u) - \Delta u \pdv{\Psi(u; \Delta u)}{u} + ... \end{gathered} \]
so
\[ \begin{aligned} &\rho(u,t) + \pdv{\rho}{t} \Delta t + (\Delta t)^2 \\ &= \int_{-\infty}^{\infty} \bqty{\rho(u,t) - \pdv{\rho}{u} \Delta u + ...}\bqty{\Psi(u; \Delta u) - \Delta u \pdv{\Psi(u; \Delta u)}{u} + ...} \dd (\Delta u )\\ &\approx \int_{-\infty}^{\infty} \pqty{\rho\Psi - \Delta u\pqty{\pdv{\rho}{u}+\pdv{\Psi}{u}} + (\Delta u)^2 \pqty{\frac12\pdv[2]{\rho}{u}\Psi + \pdv{\rho}{u}\pdv{\Psi}{u} + \frac12 \rho \pdv[2]{\Psi}{u}} }\dd(\Delta u) \end{aligned} \]
let 条件矩:
\[ a_n(u) = \int(\Delta u )^n \Psi(u;\Delta u) \dd(\Delta u) = \begin{cases} 1 &,n=0 (归一化) \\ A(u)\Delta t + o(\Delta t) &,n=1 \\ B(u)\Delta t + o(\Delta t) &,n=2 \\ o(\Delta t) &,n\ge 3 \end{cases} \]
其中漂移系数 \(A(u) = \lim_{\Delta t \to 90}\)
Chapter 9 Transition state II 自由能变化实际上是平均力势 (Potential mean force, PMF)变化。根据扩散方程:
\[ \pdv{\rho}{t} = D \pdv[2]{\rho}{x} + \frac{D}{kT}\pdv{x}\pqty{\rho\pdv{V}{x}} \]
在平衡态我们有\(\pdv{\rho}{t} = 0\) ,即通量为定值:
\[ \begin{aligned} -D \pdv{\rho}{x} -\frac{D}{kT}\pqty{\rho\pdv{V}{x}} &= j_S\\ \pdv{\rho}{x} + \frac{1}{kT}\pqty{\rho\pdv{V}{x}} &= -\frac{j_S}{D}\\ \end{aligned} \]
我们同乘一个系数 \(e^{V/kT}\) ,然后假设产物到过渡态是0到1的过程:
\[ \begin{aligned} \pdv{x}\pqty{e^{V/kT} \rho} &= -\frac{j_S}{D} e^{V/kT}\\ \rho_1(x)e^{V_1/kT} - \rho_0e^{V_0/kT} &= -\frac{j_S}{D}\int_0^1 e^{V/kT} \dd x\\ \rho_1(x)e^{V_1/kT} - \rho_0e^{V_0/kT} &= -\frac{j_S}{D} e^{V_1/kT}\int_0^1 e^{(V-V_1)/kT} \dd x \end{aligned} \]
我们可以用谐振子模拟势能,由于高斯积分主要在 \(x=1\) 附近,我们扩展乘整个实数:
\[ \begin{aligned} \rho_1(x)e^{V_1/kT} &= \rho_0e^{V_0/kT} -\frac{j_S}{D} e^{V_1/kT}\int_0^1 e^{-\omega(x-1)^2/2kT} \dd x \\ &= \rho_0e^{V_0/kT} -\frac{j_S}{D} e^{V_1/kT}\int_{-\infty}^{\infty} e^{-\omega(x-1)^2/kT} \dd x \\ \rho(x) &= \rho_0e^{(V_0 - V(x))/kT} -\frac{j_S}{D}\cdot \sqrt{\frac{2\pi kT}{\omega}} \end{aligned} \]
此处我们假设 \(V_0 = V_1 = 0\) 。
对于 \(x\to 0\) ,可以认为 \(\rho (x) \propto \rho_0 e^{\frac{V_0-V(x)}{kT}}\) ,用归一化得到:
\[ \begin{aligned} \rho_0 \int_0^1e^{(V_0 - V(x))/kT} \dd x &= 1 \\ \rho_0 \int_0^1e^{-\omega_0 x^2 / 2kT} \dd x &= 1 \\ \rho_0 &= \sqrt{\frac{\omega_0}{2\pi kT}} \end{aligned} \]
于是我们得到:
\[ \rho(x) = \sqrt{\frac{\omega_0}{2\pi kT}}e^{-\Delta V/kT} -\frac{j_S}{D}\cdot \sqrt{\frac{2\pi kT}{\omega_1}} \]
我们可以认为:前一项是由于玻尔兹曼分布的作用,后一项是由于“流动”引起的分布函数下降。
我们回归到这个流量本身。我们可以认为流量是由某种速度 \(u\) 控制的:
\[ j_S = \rho_1 u \]
这就是说:
\[ \begin{gathered} \frac{j_S}{u} + \frac{j_S}{D}\cdot \sqrt{\frac{2\pi kT}{\omega_1}}= \sqrt{\frac{\omega_0}{2\pi kT}}e^{-\Delta V/kT} \\ j_S = \frac{\sqrt{\frac{\omega_0}{2\pi kT}}}{1/(D\sqrt{\frac{\omega_1}{2\pi kT}}) + 1/u} e^{-\Delta V/kT} \end{gathered} \]
考虑 \(u \to 0\) :
\[ j_S = u\sqrt{\frac{\omega_0}{2\pi kT}}e^{-\Delta V/kT} \]
考虑 \(D\to0\) :
\[ j_S = D\frac{\sqrt{\omega_0\omega_1}}{2\pi kT}e^{-\Delta V/kT} \]
这种情况被称为扩散控制。
微扰黄金规则 假设我们有两个态 \(\ket{1}\) 和 \(\ket{0}\) :
\[ \mqty(H_0&\Delta \\ \Delta & H_1) \]
\[ H = H_0 + \lambda V(t) \]
我们知道含时薛定谔方程:
\[ i\hbar\pdv{\Psi}{t} = \hat{H} \Psi(t) \]
对于一个未微扰的系统:
\[ H_0 \Psi_m = E_m \Psi_m \]
现在我们进行微扰:
\[ \Psi(t) = \sum_m a_m(t)\Psi_m e^{-iE_mt/\hbar} \]
于是有:
\[ \begin{aligned} &\quad i\hbar \sum_m \dv{t}a_m(t)\Psi_m e^{-iE_mt/\hbar} + \sum_m a_m(t)E_m\Psi_m e^{-iE_mt/\hbar}\\ &= i\hbar \sum_m a_m(t)E_m\Psi_m e^{-iE_mt/\hbar} + \lambda\sum_m V(t)a_m(t)\Psi_m e^{-iE_mt/\hbar}\\ \end{aligned} \]
化成bra-ket形式:
\[ i\hbar \dv{a_k}{t} e^{-iE_kt/\hbar} = \lambda\sum_m a_m \mel{\Psi_k}{V}{\Psi_m}e^{-iE_mt/\hbar} \]
这就可以得到:
\[ i\hbar \dv{a_k}{t} = \lambda\sum_m H^{(1)}_{km} e^{i\omega_{km} t}\qc \omega_{km} = \frac{E_k - E_m}{\hbar} \]
我们作一阶近似,
\[ a_m(t= 0) = a_m\delta(n-m) \]
此时我们得到:
\[ i\hbar \dv{a_k}{t} = \lambda H^{(1)}_{km} e^{i\omega_{km} t} \]
积分一次:
\[ a_k^{(1)} = \frac\lambda{i\hbar} \int_0^t H_{km}^{(1)} e^{i\omega_{km} t'} \dd t' \]
然后
\[ \begin{gathered} a^{(1)}_k = -\frac{\lambda H_{km}^{(1)}}{\hbar} \frac{e^{i\omega t}-1}{\omega_{km}} \\ |a^{(1)}_k|^2 = 4|\lambda H_{km}^{(1)}|^2\frac{\sin[2](\omega_{km}t/2)}{\hbar^2 \omega_{km}^2} \end{gathered} \]
t趋于无穷时:
\[ \Gamma = |a^{(1)}_k|^2/t = \frac{2\pi}{\hbar}|H_{km}^{(1)}|^2 \delta(E_k - E_m ) \]
事实上对于电子转移理论有:
\[ \frac{1}{\sqrt{4\pi \lambda kT}}e^{-\frac{(\lambda+\Delta G)^2}{4\lambda kT}} \]
这就是Marcus理论。
对于一个电磁场,定义微扰算符:
\[ \lambda V(t) = -E_0 M\cdot \hat\epsilon \cos(\omega t) \]
相对应的:
\[ |a^{(1)}_{ki}|^2 = E_0^2 \abs{\mel{f}{\hat\epsilon\hat M}{i}}^2 \Bqty{\frac{\sin[2](\frac12(\omega_{fi} - \omega))}{\hbar^2(\omega_{fi} - \omega)^2} - \frac{\sin[2](\frac12(\omega_{fi} + \omega))}{\hbar^2(\omega_{fi} + \omega)^2}} \]
\[ P_{i\to f} (\omega) = \frac{\pi E_0^2}{2\hbar^2}\abs{\mel{f}{\hat\epsilon\hat M}{i}}^2(\delta(\omega_{fi} - \omega) +\delta(\omega_{fi} + \omega)) \]
2026年1月6日 02:44:51 2025年11月19日 18:22:00